Physicochemical Properties
| Molecular Formula | C20H24N4OS |
| Molecular Weight | 368.495762825012 |
| Exact Mass | 368.167 |
| Elemental Analysis | C, 65.19; H, 6.57; N, 15.20; O, 4.34; S, 8.70 |
| CAS # | 896657-58-2 |
| Related CAS # | 896657-58-2 |
| PubChem CID | 2993112 |
| Appearance | White to off-white solid powder |
| Density | 1.3±0.1 g/cm3 |
| Index of Refraction | 1.664 |
| LogP | 4.65 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 26 |
| Complexity | 493 |
| Defined Atom Stereocenter Count | 0 |
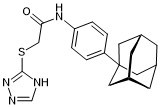
| SMILES | O=C(CSC1NC=NN=1)NC1C=CC(C23CC4CC(C2)CC(C4)C3)=CC=1 |
| InChi Key | UUKXOVBCMOZQNC-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C20H24N4OS/c25-18(11-26-19-21-12-22-24-19)23-17-3-1-16(2-4-17)20-8-13-5-14(9-20)7-15(6-13)10-20/h1-4,12-15H,5-11H2,(H,23,25)(H,21,22,24) |
| Chemical Name | N-[4-(1-adamantyl)phenyl]-2-(1H-1,2,4-triazol-5-ylsulfanyl)acetamide |
| Synonyms | MGH CP1; MGH-CP1; MGHCP1 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | TEAD2 (IC50 = 710 nM); TEAD4 (IC50 = 672 nM) |
| ln Vitro | In vitro, MGH-CP1 shows a dose-dependent and strong inhibition of TEAD2/4 auto-palmitoylation. Treatment with MGH-CP1 significantly lowers the levels of palmitoylation of native or ectopically expressed TEAD proteins in cells. MGH-CP1 is selective for TEADs because it has no effect on the auto-palmitoylation of a number of palmitoyl acyltransferases from the ZDHHC family. By directly focusing on TEAD auto-palmitoylation, MGH-CP1 is a selective small-molecule pan-TEAD inhibitor. [1] |
| ln Vivo | In vivo, MGH-CP1 reduces TEAD activity in the intestine of Lats1/2 KO mice. The palmitoylation of TEAD proteins in the intestinal epithelium can be effectively inhibited by MGH-CP1. After two weeks of treatment, MGH-CP1 is well tolerated and appears to have no negative effects on the general health or body weight of the animal. The TEAD target genes CTGF and ANKRD1 are effectively inhibited by MGH-CP1 treatment in Lats1/2 KO intestine, in contrast to its apparent lack of effect in wild-type intestine.[1] |
| Cell Assay | In DMEM supplemented with 10% FBS and 1% penicillin/streptomycin, HEK293T cells, Lats1/2 conditional MEFs, and MDA-MB-231 cells are cultured. Prior to the next experiment, Lats1/2 is deleted in Lats1/2 conditional MEFs carrying CMV-CreER by incubating the cells with 4-OH Tamoxifen (2.5 mM) in DMEM for 4 days. Lipofectamine 2000 is used for transfection in HEK293T cells. For luciferase reporter assays, HEK293T cells are transfected with the expression vectors of the following proteins: pCIG-Wnt3a, pCMV-LRP5C, pCIG-BMP4, pCIG-Gli1, pGIPZ-IKBKE, and pCMV-Renilla lucifease. Using the dual-lucif-erase reporter kit, luciferase activities are detected 24 hours after transfection in cells treated with or without Wnt3A, LiCl, or MGH-CP1. |
| References |
[1]. Cell Stem Cell . 2020 May 7;26(5):675-692.e8. |
Solubility Data
| Solubility (In Vitro) |
DMSO: 74~100 mg/mL (200.8~271.4 mM) Ethanol: ~74 mg/mL (~200.8 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.78 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.78 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (6.78 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7137 mL | 13.5685 mL | 27.1370 mL | |
| 5 mM | 0.5427 mL | 2.7137 mL | 5.4274 mL | |
| 10 mM | 0.2714 mL | 1.3569 mL | 2.7137 mL |