Physicochemical Properties
| Molecular Formula | C47H64CLN5O13S |
| Molecular Weight | 974.55 |
| Exact Mass | 973.39 |
| CAS # | 1100692-14-5 |
| PubChem CID | 166596981 |
| Appearance | White to off-white solid powder |
| LogP | 2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 13 |
| Heavy Atom Count | 67 |
| Complexity | 1950 |
| Defined Atom Stereocenter Count | 8 |
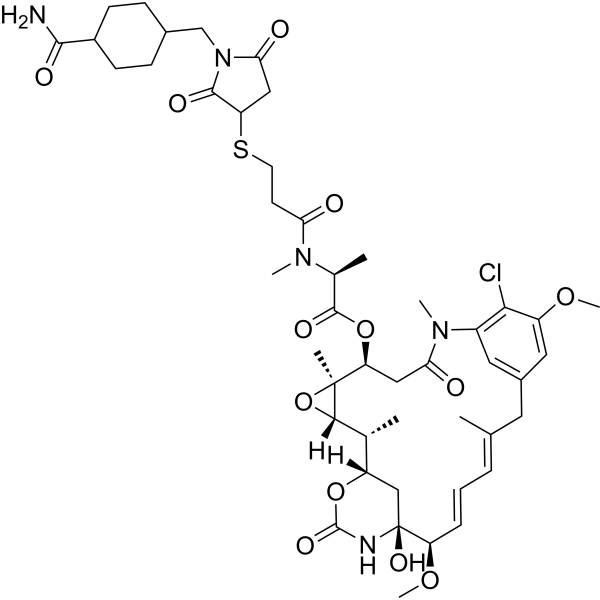
| SMILES | C[C@]12[C@]([H])(CC(N(C3=C(C(OC)=CC(CC(C)=CC=C[C@@]([H])(OC)[C@@]4(NC(=O)O[C@@]([H])(C4)[C@@H](C)[C@@H]1O2)O)=C3)Cl)C)=O)OC(=O)[C@H](C)N(C)C(=O)CCSC1CC(=O)N(CC2CCC(C(=O)N)CC2)C1=O |t:16,18,&1:1,2,20,24,29,32,34,44| |
| InChi Key | WPWQMVXPTHKASL-KLVLVJRKSA-N |
| InChi Code | InChI=1S/C47H64ClN5O13S/c1-25-10-9-11-35(63-8)47(61)23-33(64-45(60)50-47)26(2)41-46(4,66-41)36(22-38(55)52(6)31-19-29(18-25)20-32(62-7)40(31)48)65-44(59)27(3)51(5)37(54)16-17-67-34-21-39(56)53(43(34)58)24-28-12-14-30(15-13-28)42(49)57/h9-11,19-20,26-28,30,33-36,41,61H,12-18,21-24H2,1-8H3,(H2,49,57)(H,50,60)/b11-9-,25-10-/t26-,27+,28?,30?,33+,34?,35-,36+,41+,46+,47+/m1/s1 |
| Chemical Name | [(1S,2R,3S,5S,6S,16Z,18Z,20R,21S)-11-chloro-21-hydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-8,23-dioxo-4,24-dioxa-9,22-diazatetracyclo[19.3.1.110,14.03,5]hexacosa-10,12,14(26),16,18-pentaen-6-yl] (2S)-2-[3-[1-[(4-carbamoylcyclohexyl)methyl]-2,5-dioxopyrrolidin-3-yl]sulfanylpropanoyl-methylamino]propanoate |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of trastuzumab emtansine during breastfeeding. Because trastuzumab is a large protein molecule with a molecular weight of 145,531 Da, the amount in milk is likely to be very low. It is also likely to be partially destroyed in the infant's gastrointestinal tract and absorption by the infant is probably minimal. However, emtansine (DM1) is a small-molecule microtubule inhibitory drug, which might be excreted into milk. The manufacturer recommends that breastfeeding be discontinued during trastuzumab deruxtecan therapy and for 7 months after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. ◉ Summary of Use during Lactation No information is available on the clinical use of ado-trastuzumab during breastfeeding. Because trastuzumab is a large protein molecule with a molecular weight of 145,531 Da, the amount in milk is likely to be very low. It is also likely to be partially destroyed in the infant's gastrointestinal tract and absorption by the infant is probably minimal. However, ado-trastuzumab emtansine also contains DM1, which is a small-molecule toxin that might enter milk and be absorbed by the infant. Because of the potential for serious adverse reactions in the breastfed infant, the manufacturer recommends avoiding breastfeeding during and for 7 months following ado-trastuzumab emtansine therapy. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| References |
[1]. Antibody-DM1 conjugates as cancer therapeutics. Cancer Lett. 2011 Aug 28;307(2):113-8. [2]. LC-MS/MS method for the simultaneous determination of Lys-MCC-DM1, MCC-DM1 and DM1 as potential intracellular catabolites of the antibody-drug conjugate trastuzumab emtansine (T-DM1). J Pharm Biomed Anal. 2017 Apr 15;137:170-177. |
| Additional Infomation |
Immunotoxin that consists of humanized monoclonal anti-HER2 antibody TRASTUZUMAB covalently linked to anti-microtubule agent MAYTANSINOID DM1 for treatment of metastatic breast cancer in patients who previously received trastuzumab and a TAXANES, separately or in combination. See also: Trastuzumab Emtansine (annotation moved to). Drug Indication Early Breast Cancer (EBC)Kadcyla, as a single agent, is indicated for the adjuvant treatment of adult patients with HER2-positive early breast cancer who have residual invasive disease, in the breast and/or lymph nodes, after neoadjuvant taxane-based and HER2-targeted therapy. Metastatic Breast Cancer (MBC)Kadcyla, as a single agent, is indicated for the treatment of adult patients with HER2-positive, unresectable locally advanced or metastatic breast cancer who previously received trastuzumab and a taxane, separately or in combination. Patients should have either: Received prior therapy for locally advanced or metastatic disease, orDeveloped disease recurrence during or within six months of completing adjuvant therapy. |
Solubility Data
| Solubility (In Vitro) | DMSO : 190 mg/mL (194.96 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 4.75 mg/mL (4.87 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 47.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 4.75 mg/mL (4.87 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 47.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 4.75 mg/mL (4.87 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 47.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.0261 mL | 5.1306 mL | 10.2611 mL | |
| 5 mM | 0.2052 mL | 1.0261 mL | 2.0522 mL | |
| 10 mM | 0.1026 mL | 0.5131 mL | 1.0261 mL |