MC1568 is a hydroxyamide-based inhibitor of class II histone deacetylase (HDAC) such as HDAC4/5/6/7/9 (IIa) and HDAC6/10 (IIb) with potential antiviral activity. It blocks the influenza A virus from multiplying.
Physicochemical Properties
| Molecular Formula | C17H15FN2O3 | |
| Molecular Weight | 314.31 | |
| Exact Mass | 314.106 | |
| Elemental Analysis | C, 64.96; H, 4.81; F, 6.04; N, 8.91; O, 15.27 | |
| CAS # | 852475-26-4 | |
| Related CAS # |
|
|
| PubChem CID | 11381449 | |
| Appearance | Pink to red solid powder | |
| Density | 1.2±0.1 g/cm3 | |
| Index of Refraction | 1.572 | |
| LogP | 2.91 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 4 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 23 | |
| Complexity | 492 | |
| Defined Atom Stereocenter Count | 0 | |
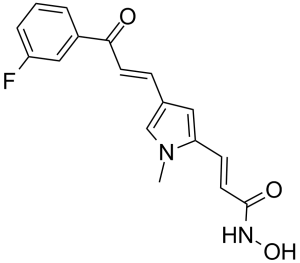
| SMILES | C(/C1=CC=C(/C=C/C(=O)NO)N1C)=C\C(C1C=CC=C(F)C=1)=O |
|
| InChi Key | QRDAPCMJAOQZSU-KQQUZDAGSA-N | |
| InChi Code | InChI=1S/C17H15FN2O3/c1-20-11-12(9-15(20)6-8-17(22)19-23)5-7-16(21)13-3-2-4-14(18)10-13/h2-11,23H,1H3,(H,19,22)/b7-5+,8-6+ | |
| Chemical Name | (E)-3-[4-[(E)-3-(3-fluorophenyl)-3-oxoprop-1-enyl]-1-methylpyrrol-2-yl]-N-hydroxyprop-2-enamide | |
| Synonyms | MC1568; MC 1568; MC-1568 | |
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | HD1-A ( IC50 = 100 nM ); HD1-B ( IC50 = 3.4 μM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | Scintillation counting is used to measure the amount of tritiated acetic acid that the enzyme extracts from the substrate. Triple determinations produce IC50 values. Ten microliters of total [3H]acetate-prelabeled chicken reticulocyte histones (2 mg/mL) are incubated for 30 minutes with a 50 μL sample of maize enzyme at 30 °C. To halt the reaction, add 800 μL of ethyl acetate and 50 μL of 1 M HCl/0.4 M acetate. A 600 μL aliquot of the upper phase is centrifuged at 1×104 g for 5 minutes, and its radioactivity is measured in 3 mL of liquid scintillation cocktail. Active ingredients are further diluted after MC1568 is tested at a starting concentration of 40 μM. The reference compounds are NaB, VPA, TSA, SAHA, 85 TPX, HC-toxin, and tubacin; the negative controls are blank solvents. | |
| Cell Assay | Using a combination of insulin, dexamethasone, and isobutylmethylxanthine, 3T3-L1 cells are grown and differentiated. During the eight-day differentiation period starting on the second day after confluence, the 3T3-L1 cells are stimulated by: (1) No induction: the cells are cultured with DMSO or MC1568 at post-confluence and for the entire 8-day differentiation period. (2) Troglitazone: the cells are induced with 5 μM troglitazone, MC1568, or both at post-confluence and for the duration of the 8-day differentiation period. (3) Rosiglitazone: the cells are cultured with 1 μM rosiglitazone and either DMSO or MC1568 at post-confluence and for the duration of the 8-day differentiation period. (4) Dexamethasone and rosiglitazone: the cells were given 390 ng/mL dexamethasone and 1 μM rosiglitazone at post-confluence. The cells are induced with 1 μM rosiglitazone and either DMSO or MC1568 for the duration of the 8-day differentiation period. Every two days, all media are refreshed. | |
| Animal Protocol |
|
|
| References |
[1]. J Med Chem . 2005 May 5;48(9):3344-53. [2]. Mol Cancer Res . 2008 Dec;6(12):1908-19. [3]. EMBO Rep . 2009 Jul;10(7):776-82. |
|
| Additional Infomation | 3-[4-[3-(3-fluorophenyl)-3-oxoprop-1-enyl]-1-methyl-2-pyrrolyl]-N-hydroxy-2-propenamide is a carbonyl compound. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 1 mg/mL (3.18 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 0.5% CMC: 5mg/mL Solubility in Formulation 3: 2.5 mg/mL (7.95 mM) in 17% Polyethylene glycol 12-hydroxystearate in Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1816 mL | 15.9079 mL | 31.8157 mL | |
| 5 mM | 0.6363 mL | 3.1816 mL | 6.3631 mL | |
| 10 mM | 0.3182 mL | 1.5908 mL | 3.1816 mL |