Physicochemical Properties
| Molecular Formula | C15H12O4 |
| Molecular Weight | 256.25 |
| Exact Mass | 256.073 |
| CAS # | 578-86-9 |
| Related CAS # | (±)-Liquiritigenin;69097-97-8 |
| PubChem CID | 114829 |
| Appearance | White to light yellow solid powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 529.5±50.0 °C at 760 mmHg |
| Melting Point | 206-208ºC |
| Flash Point | 206.9±23.6 °C |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.662 |
| LogP | 2.76 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Heavy Atom Count | 19 |
| Complexity | 335 |
| Defined Atom Stereocenter Count | 1 |
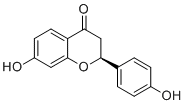
| SMILES | C1[C@H](OC2=C(C1=O)C=CC(=C2)O)C3=CC=C(C=C3)O |
| InChi Key | FURUXTVZLHCCNA-AWEZNQCLSA-N |
| InChi Code | InChI=1S/C15H12O4/c16-10-3-1-9(2-4-10)14-8-13(18)12-6-5-11(17)7-15(12)19-14/h1-7,14,16-17H,8H2/t14-/m0/s1 |
| Chemical Name | (S)-7-hydroxy-2-(4-hydroxyphenyl)chroman-4-one |
| Synonyms | MenerbaLiquiritigenin MF101 MF 101 MF-101 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Liquiritigenin transfects U2OS cells with ERβ but not ERα, and this results in dose-responsive activation of ERE tk-Luc. Liquiritigenin caused temporal excitation of CECR6, NKG2E, and NKD in addition to dosage activation of ERβ but not ERα. Liquiritigenin's ERβ selectivity results from the coactivator shock coactivator-like 2 being selectively recruited to target genes. Liquiritigenin binds to ERα and ERβ with comparable affinities, which leads to the selective recruitment of SRC-2 to target genes in ERβ cells [Liquiritigenin MC3T3-E1 cells inhibits the production of TNF-α, intracellular reactive oxygen species, protein adducts, MG-induced osteoblast MC3T3-E1 death, mitochondrial superoxide, and cardiolipin peroxidation [1]. 2]. |
| ln Vivo | In a mouse xenograft model, liquiditrigenin did not increase the size of the epidermis or cause carcinogenesis in MCF-7 breast cancer cells [1]. Treatment with lichidetinin has the ability to markedly lower serum and hippocampal concentrations of pro-inflammatory cytokines, including as interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α [3]. |
| References |
[1]. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol Cell Endocrinol. 2008 Feb 13;283(1-2):49-57. [2]. Protective effect of liquiritigenin against methylglyoxal cytotoxicity in osteoblastic MC3T3-E1 cells. Food Funct. 2014 Jul 25;5(7):1432-40. [3]. Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav Brain Res. 2016 Jul 15;308:177-86. |
| Additional Infomation |
Liquiritigenin is a dihydroxyflavanone compound having the two hydroxy substituents at the 4'- and 7-positions. Isolated from the root of Glycyrrhizae uralensis, it is a selective agonist for oestrogen receptor beta. It has a role as a hormone agonist and a plant metabolite. 5-deoxyflavanone is a solid. This compound belongs to the flavanones. These are compounds containing a flavan-3-one moiety, whose structure is characterized by a 2-phenyl-3,4-dihydro-2H-1-benzopyran bearing a ketone at the carbon C3. MF101 is a novel estrogen receptor beta (ERβ) selective agonist and unlike currently available hormone therapies, does not activate the estrogen receptor alpha (ERα), known to be implicated in tumor formation. MF101 is an oral drug designed for the treatment of hot flashes and night sweats in peri-menopausal and menopausal women. Liquiritigenin has been reported in Glycyrrhiza pallidiflora, Hedysarum polybotrys, and other organisms with data available. See also: Glycyrrhiza Glabra (part of); Glycyrrhiza uralensis Root (part of); Pterocarpus marsupium wood (part of). Drug Indication Investigated for use/treatment in hormone replacement therapy: menopause and menopause. Mechanism of Action MF101 promoted ERbeta, but not ERalpha, activation of an estrogen response element (ERE) upstream of the luciferase reporter gene. MF101 also selectively regulates transcription of endogenous genes through ERbeta. The ERbeta-selectivity was not due to differential binding, since MF101 binds equally to ERalpha and ERbeta. Fluorescence resonance energy transfer and protease digestion studies showed that MF101 produces a different conformation in ERalpha from ERbeta, when compared with the conformations produced by estradiol. The specific conformational change induced by MF101 allows ERbeta to bind to an ERE and recruit coregulatory proteins that are required for gene activation. MF101 did not activate the ERalpha-regulated proliferative genes, c-myc and cyclin D1, or stimulate MCF-7 breast cancer cell proliferation or tumor formation in a mouse xenograft model. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~125 mg/mL (~487.80 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.42 mg/mL (9.44 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 24.2 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.42 mg/mL (9.44 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 24.2 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.42 mg/mL (9.44 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 24.2 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9024 mL | 19.5122 mL | 39.0244 mL | |
| 5 mM | 0.7805 mL | 3.9024 mL | 7.8049 mL | |
| 10 mM | 0.3902 mL | 1.9512 mL | 3.9024 mL |