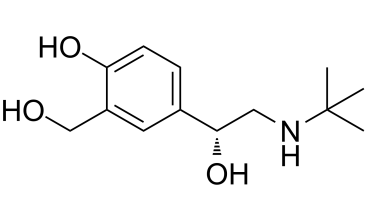
Levalbuterol, (R)-albuterol or the R-isomer of albuterol, is a potent β2-adrenergic receptor agonist used to treat asthma and COPD-chronic obstructive pulmonary disease.
Physicochemical Properties
| Molecular Formula | C₁₃H₂₁NO₃ |
| Molecular Weight | 239.31 |
| Exact Mass | 239.152 |
| CAS # | 34391-04-3 |
| Related CAS # | Levalbuterol hydrochloride; 50293-90-8; Levalbuterol tartrate; 661464-94-4 |
| PubChem CID | 123600 |
| Appearance | Typically exists as solid at room temperature |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 433.5±40.0 °C at 760 mmHg |
| Flash Point | 159.5±17.9 °C |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.566 |
| LogP | 0.01 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 17 |
| Complexity | 227 |
| Defined Atom Stereocenter Count | 1 |
| SMILES | CC(C)(C)NC[C@@H](C1=CC(=C(C=C1)O)CO)O |
| InChi Key | NDAUXUAQIAJITI-LBPRGKRZSA-N |
| InChi Code | InChI=1S/C13H21NO3/c1-13(2,3)14-7-12(17)9-4-5-11(16)10(6-9)8-15/h4-6,12,14-17H,7-8H2,1-3H3/t12-/m0/s1 |
| Chemical Name | 4-[(1R)-2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)phenol |
| Synonyms | Levalbuterol; (R)-albuterol; Xopenex; R-albuterol; Levosalbutamol |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro |
Levalbuterol (10 μM; 24 hours) causes 11β-HSD1 mRNA expression in airway epithelial cells, but not 11β-HSD2 expression[1]. Levalbuterol (10 μM; 24 hours) increases GRE activation in an 11β-HSD1 dependent manner in a transformed mouse airway epithelial cell line, while significantly reducing both LPS- and TNF-α-induced NF-κB activity[1]. |
| ln Vivo | Levalbuterol (subcutaneous injection; 1 mg/kg; 14 days) dramatically reduces pulmonary inflammation in OVA mice, as evidenced by a drop in IgE and eosinophilia[3]. |
| Cell Assay |
Cell Line: Murine Club (MTCC) cells Concentration: 10 μM Incubation Time: 24 hours Result: Increased 11β-HSD1 mRNA expression selectively. |
| Animal Protocol |
C57BL/6 female mice with a pulmonary allergic model 1 mg/kg Subcutaneous injection; 1 mg/kg; 14 days |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Inhalation delivers the medication directly into the airways and lungs, thereby minimizing side effects because of reduced systemic absorption of the inhaled medications. excreted into the urine. Metabolism / Metabolites Pure (R)-salbutamol formulation known as levosalbutamol is metabolised up to 12 times faster than (S)-salbutamol by intestine. Biological Half-Life 3.3 - 4 hours |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Levalbuterol is the R-enantiomer of the beta-2 adrenergic agonist, albuterol (salbutamol). Although no published data exist on the use of levalbuterol by mouth or inhaler during lactation, data from the related drug, terbutaline, indicate that very little is expected to be excreted into breastmilk. The authors of several reviews and expert guidelines agree that use of inhaled bronchodilators is acceptable during breastfeeding because of the low bioavailability and maternal serum levels after use. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding plasma protein binding is relatively low. |
| References |
[1]. Anti-inflammatory effects of levalbuterol-induced 11β-hydroxysteroid dehydrogenase type 1 activity in airway epithelial cells.Front Endocrinol (Lausanne). 2015 Jan 12;5:236. [2]. (R)-albuterol decreases immune responses: role of activated T cells.Respir Res. 2008 Jan 14;9:3. |
| Additional Infomation |
(R)-salbutamol is an albuterol. Levosalbutamol, or levalbuterol, is a short-acting β2 adrenergic receptor agonist used in the treatment of asthma and chronic obstructive pulmonary disease (COPD). [Salbutamol] has been marketed as a racemic mixture, although beta2-agonist activity resides almost exclusively in the (R)-enantiomer. The enantioselective disposition of salbutamol and the possibility that (S)-salbutamol has adverse effects have led to the development of an enantiomerically pure (R)-salbutamol formulation known as levosalbutamol (levalbuterol). Levalbuterol is a beta2-Adrenergic Agonist. The mechanism of action of levalbuterol is as an Adrenergic beta2-Agonist. Levalbuterol is a short-acting sympathomimetic beta-2 adrenergic receptor agonist with bronchodilator activity. Levalbuterol binds to beta-2 adrenergic receptors in bronchial smooth muscle and activates intracellular adenyl cyclase, an enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3',5'-adenosine monophosphate (cAMP). Increased cAMP levels lead to the activation of protein kinase A, which inhibits the phosphorylation of myosin and lowers intracellular ionic calcium concentrations, resulting in relaxation of bronchial smooth muscles. The increased cAMP concentrations also inhibit the release of inflammatory mediators, especially from mast cells. The R-isomer of albuterol. See also: Levalbuterol Hydrochloride (has salt form); Levalbuterol Tartrate (has salt form); Levalbuterol Sulfate (has salt form) ... View More ... Drug Indication Indicated for the management of COPD (chronic obstructive pulmonary disease, also known as chronic obstructive lung disease) and asthma. Mechanism of Action β2 adrenergic receptors on airway smooth muscle are Gs coupled and their activation by levosalbutamol leads to activation of adenylate cyclase and to an increase in the intracellular concentration of 3',5'-cyclic adenosine monophosphate (cyclic AMP). Increased cyclic AMP activates protein kinase A which itself inhibits the phosphorylation of myosin produces lower intracellular ionic calcium concentrations, inducing muscle relaxation. Increased cyclic AMP concentrations are also associated with the inhibition of the release of mediators from mast cells in the airways, potentially contributing to its benefit in asthma attacks. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1787 mL | 20.8934 mL | 41.7868 mL | |
| 5 mM | 0.8357 mL | 4.1787 mL | 8.3574 mL | |
| 10 mM | 0.4179 mL | 2.0893 mL | 4.1787 mL |