Physicochemical Properties
| Molecular Formula | C53H60F2N8O12 |
| Molecular Weight | 1039.09 |
| Exact Mass | 1038.43 |
| CAS # | 1502654-87-6 |
| Related CAS # | Ledipasvir;1256388-51-8;Ledipasvir (acetone);1441674-54-9;Ledipasvir-d6;2050041-12-6;Ledipasvir hydrochloride;2128695-48-5;Ledipasvir (diacetone);1502655-48-2 |
| PubChem CID | 78357794 |
| Appearance | Off-white to yellow solid powder |
| LogP | 7.142 |
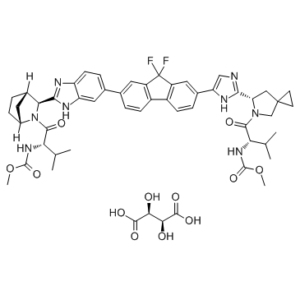
| SMILES | CC(C)[C@@H](C(=O)N1CC2(CC2)C[C@H]1C3=NC=C(N3)C4=CC5=C(C=C4)C6=C(C5(F)F)C=C(C=C6)C7=CC8=C(C=C7)N=C(N8)[C@@H]9[C@H]1CC[C@H](C1)N9C(=O)[C@H](C(C)C)NC(=O)OC)NC(=O)OC.[C@H]([C@@H](C(=O)O)O)(C(=O)O)O |
| InChi Key | ZQVLPYMRXLPMDX-KEAIDYLOSA-N |
| InChi Code | InChI=1S/C49H54F2N8O6.C4H6O6/c1-24(2)39(56-46(62)64-5)44(60)58-23-48(15-16-48)21-38(58)42-52-22-37(55-42)28-9-13-32-31-12-8-26(18-33(31)49(50,51)34(32)19-28)27-10-14-35-36(20-27)54-43(53-35)41-29-7-11-30(17-29)59(41)45(61)40(25(3)4)57-47(63)65-6;5-1(3(7)8)2(6)4(9)10/h8-10,12-14,18-20,22,24-25,29-30,38-41H,7,11,15-17,21,23H2,1-6H3,(H,52,55)(H,53,54)(H,56,62)(H,57,63);1-2,5-6H,(H,7,8)(H,9,10)/t29-,30+,38-,39-,40-,41-;1-,2-/m00/s1 |
| Chemical Name | Methyl N-[(2S)-1-[(6S)-6-[5-[9,9-Difluoro-7-[2-[(1S,2S,4R)-3-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]-3-azabicyclo[2.2.1]heptan-2-yl]-3H-benzimidazol-5-yl]fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspiro[2.4]heptan-5-yl]-3-methyl-1-oxobutan-2-yl]carbamate tartrate |
| Synonyms | GS-5885 tartrate, GS5885 tartrate; GS 5885; Ledipasvir D-tartrate; Ledipasvir (D-tartrate); 1502654-87-6; RT680T6HCQ; 1499193-68-8; UNII-RT680T6HCQ; (2S,3S)-2,3-dihydroxybutanedioic acid;methyl N-[(2S)-1-[(6S)-6-[5-[9,9-difluoro-7-[2-[(1R,3S,4S)-2-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]-2-azabicyclo[2.2.1]heptan-3-yl]-3H-benzimidazol-5-yl]fluoren-2-yl]-1H-imidazol-2-yl]-5-azaspiro[2.4]heptan-5-yl]-3-methyl-1-oxobutan-2-yl]carbamate; trade name: Harvoni; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | GT1a(EC50=34 pM);GT1b(EC50=4 pM) | ||
| ln Vitro | Ledipasvir (also known as GS5885) is a HCV NS5A polymerase inhibitor that is used for the treatment of hepatitis C virus infection. The combination product of ledipasvir 90 mg/sofosbuvir 400 mg (trade name Harvoni) was approved by FDA in October 2014. The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin. Ledipasvir has an extended plasma half-life of 37-45 h in healthy volunteers and produces a rapid >3 log viral load reduction in monotherapy at oral doses of 3 mg or greater with once-daily dosing in genotype 1a HCV-infected patients. It has been shown to be safe and efficacious, with SVR12 rates up to 100% when used in combination with direct-acting antivirals having complementary mechanisms. | ||
| ln Vivo | In clinical trials, it was observed ledpasvir was well tolerated and exhibited median maximal reduction of HCV RNA ranging from 2.3 log10 IU/ml to 3.3 log10 IU/ml. Emax modeling also showed administration of 30 mg ledpasvir after 3 days resulted in >95% maximal response of HCV RNA reduction to genotype 1a.Finally, it was also observed that HCV RNA was more sustained in genotype 1b compared to 1a. | ||
| Enzyme Assay |
Competitive Protein Binding Assay[1] Human plasma and cell-culture medium containing 10% fetal bovine serum (CCM) were spiked with the test compound at a final concentration of 2 μM. Spiked plasma (1 mL) and CCM (1 mL) were placed into opposite sides of the assembled dialysis cells, which are separated by a semipermeable membrane. The dialysis cells were rotated slowly in a 37 °C water bath for the time necessary to reach equilibrium. Postdialysis plasma and CCM weights were measured, and the test compound concentrations in plasma and CCM were determined with LC/MS/MS. Metabolic Stability[1] Metabolic stability in vitro was determined using pooled hepatic microsomal fractions (final protein concentration of 0.5 mg/mL) at a final test compound concentration of 3 μM. The reaction was initiated by the addition of an NADPH-regenerating system. Aliquot of 25 μL of the reaction mixture were transferred at various time points to plates containing a quenching solution. The test compound concentration in the reaction mixture was determined with LC/MS/MS. Hepatic intrinsic clearance was calculated as described previously by Obach, and the predicted clearance was calculated using the well-stirred liver model without protein restriction. Metabolic stability was also determined in cryopreserved hepatocytes using tritiated test compounds. The incubation mixture contained 1 × 106 hepatocytes/mL and 1 μM tritiated test compound (2.5 μCi). The incubation was carried out with gentle shaking at 37 °C under a humid atmosphere of 95% air/5% CO2 (v/v). Aliquots of 50 μL were removed after 0, 1, 3, and 6 h and added to 100 μL of quenching solution. The samples were analyzed on a flow scintillation radio detector coupled to an HPLC system. The metabolites were quantified on the basis of the peak areas from the radio detector, with the cell-free control samples used as a reference. Metabolic stabilities in hepatocytes were determined by measuring the rate of disappearance of the test compound as the percent of total peak areas of the formed radiolabeled metabolites and the test compound. |
||
| Cell Assay | Ledipasvir is a specific inhibitor of HCV NS5A protein to inhibit HCV replication in the HCV subgenomic replicon system. NS5A replication complex inhibitors are novel antiviral factors for HCV treatment. Typically, these inhibitors have high efficiency and low viral resistance when compared to traditional HCV replication inhibitor targeted on NS3 helicase and NS5B RNA polymerasae. NS5A inhibitors are supposed to bind across the NS5A dimer interface, proximal to N-terminal domain 1. The binding is thought to distort dimer association directly or allosterically, which may disrupt NS5A function in HCV RNA replication. When a JFH1/3a-NS5A hybrid replicon was used to assess susceptibility to NS5A, another inhibitor DCV was shown to be more potent than ledipasvir. Additionally, NS5A-A30K and -Y93H variants exhibited reduced sensitivity to ledpasvir (EC50 value of 1770 nM and 4300 nM respectively). | ||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Absorption When given orally, ledipasvir reaches its maximum plasma concentration in about 4 to 4.5 hours with a maximum concentration (Cmax) of 323 ng/mL. Route of Elimination Following a single 90 mg oral dose of [14C]-ledipasvir, mean total recovery of the [14C]-radioactivity in feces and urine was approximately 87%, with most of the radioactive dose recovered from feces (approximately 86%). Unchanged ledipasvir excreted in feces accounted for a mean of 70% of the administered dose and the oxidative metabolite M19 accounted for 2.2% of the dose. These data indicate that biliary excretion of unchanged ledipasvir is a major route of elimination, with renal excretion being a minor pathway (approximately 1%). Metabolism / Metabolites In vitro, no detectable metabolism of ledipasvir was observed by human CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Evidence of slow oxidative metabolism via an unknown mechanism has been observed. Following a single dose of 90 mg [14C]-ledipasvir, systemic exposure was almost exclusively to the parent drug (>98%). Unchanged ledipasvir is the major species present in feces. Biological Half-Life The median terminal half-life of ledipasvir is 47 hours. |
||
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Ledipasvir has not been studied in nursing mothers being treated for hepatitis C infection. Because it is 99.8% bound to maternal plasma proteins, amounts in breastmilk are likely to be very low. If ledipasvir alone or in combination with sofosbuvir (Harvoni) is required by the mother, it is not a reason to discontinue breastfeeding. Some sources recommend against breastfeeding when ledipasvir is used with ribavirin. Hepatitis C is not transmitted through breastmilk and breastmilk has been shown to inactivate hepatitis C virus (HCV). However, the Centers for Disease Control recommends that mothers with HCV infection should consider abstaining from breastfeeding if their nipples are cracked or bleeding. It is not clear if this warning would apply to mothers who are being treated for hepatitis C. Infants born to mothers with HCV infection should be tested for HCV infection; because maternal antibody is present for the first 18 months of life and before the infant mounts an immunologic response, nucleic acid testing is recommended. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Drugs and Lactation Database (LactMed) Protein Binding Ledipasvir is >99.8% bound to human plasma proteins. |
||
| References |
[1]. Discovery of ledipasvir (GS-5885): a potent, once-daily oral NS5A inhibitor for the treatment of hepatitis C virus infection. J Med Chem. 2014 Mar 13;57(5):2033-46 [2]. Natural prevalence of NS5A polymorphisms in subjects infected with hepatitis C virus genotype 3 and their effects on the antiviral activity of NS5A inhibitors. J Clin Virol. 2013 May;57(1):13-8. |
||
| Additional Infomation |
A new class of highly potent NS5A inhibitors with an unsymmetric benzimidazole-difluorofluorene-imidazole core and distal [2.2.1]azabicyclic ring system was discovered. Optimization of antiviral potency and pharmacokinetics led to the identification of 39 (ledipasvir, GS-5885). Compound 39 (GT1a replicon EC50 = 31 pM) has an extended plasma half-life of 37-45 h in healthy volunteers and produces a rapid >3 log viral load reduction in monotherapy at oral doses of 3 mg or greater with once-daily dosing in genotype 1a HCV-infected patients. 39 has been shown to be safe and efficacious, with SVR12 rates up to 100% when used in combination with direct-acting antivirals having complementary mechanisms.[1] Background: Hepatitis C virus (HCV) NS5A replication complex inhibitors (RCIs) have been shown to exhibit picomolar antiviral activity against genotype 1 (GT1) in vitro. This has translated into rapid and robust declines in HCV RNA in GT1 patients. Less is known about the susceptibility of other genotypes such as GT3 to inhibition by NS5A RCIs. Objectives: To detect and phenotype naturally occurring HCVGT3 NS5A polymorphisms against two NS5A RCIs (daclatasvir [DCV] and GS-5885) currently in clinical development. Study design: The NS5A region from 96 HCV GT3 treatment-naive patients spanning North America, Europe and Australia was determined. Results: Phylogenetic analysis revealed a broad distribution with no significant geographic clustering. GT1 DCV resistance-associated variants (RAVs) were observed in GT3 subjects; variants (and their frequencies) included 28M/V (1%), 30A/K/S/T/V (10%), 31L/M (1%), E92A (1%) and Y93H (8.3%). A consensus sequence was used to generate a JFH1/3a-NS5A hybrid replicon and employed to assess susceptibility to NS5A RCIs. Against JFH1/3a-NS5A, DCV was more potent (EC(50) = 0.52 nM) than GS-5885 (EC(50) = 141 nM). DCV sensitivity was increased against JFH1/3a-NS5A-M28V (EC50 = 0.006 nM), A30V (EC(50) = 0.012 nM), and E92A (EC(50) = 0.004 nM) while the NS5A-A30K and -Y93H variants exhibited reduced sensitivity to DCV (EC50 values of 23 nM and 1120 nM, respectively) and to GS-5885 (EC50 values of 1770 nM and 4300 nM, respectively). Conclusions: Substitutions conferring resistance to NS5A RCIs pre-existed in treatment-naive patients infected with HCV GT3. The effectiveness of these NS5A RCIs to exert efficacy in the clinic may depend on which inhibitor is used in combination with other antivirals.[2] |
Solubility Data
| Solubility (In Vitro) | DMSO : ~25 mg/mL (~24.06 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (2.41 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (2.41 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 3: 10% DMSO+90% Corn Oil: ≥ 2.5 mg/mL (2.41 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.9624 mL | 4.8119 mL | 9.6238 mL | |
| 5 mM | 0.1925 mL | 0.9624 mL | 1.9248 mL | |
| 10 mM | 0.0962 mL | 0.4812 mL | 0.9624 mL |