LP-261 is a novel and potent mitotic/tubulin inhibitor with potential anticaner activities. Cell cycle arrest at G2/M phase is achieved by blocking tubulin polymerization.
Physicochemical Properties
| Molecular Formula | C22H19N3O4S |
| Molecular Weight | 421.47 |
| Exact Mass | 421.11 |
| Elemental Analysis | C, 62.69; H, 4.54; N, 9.97; O, 15.18; S, 7.61 |
| CAS # | 915412-67-8 |
| Related CAS # | 915412-67-8 |
| PubChem CID | 15603282 |
| Appearance | White to off-white solid powder |
| LogP | 4.994 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 30 |
| Complexity | 710 |
| Defined Atom Stereocenter Count | 0 |
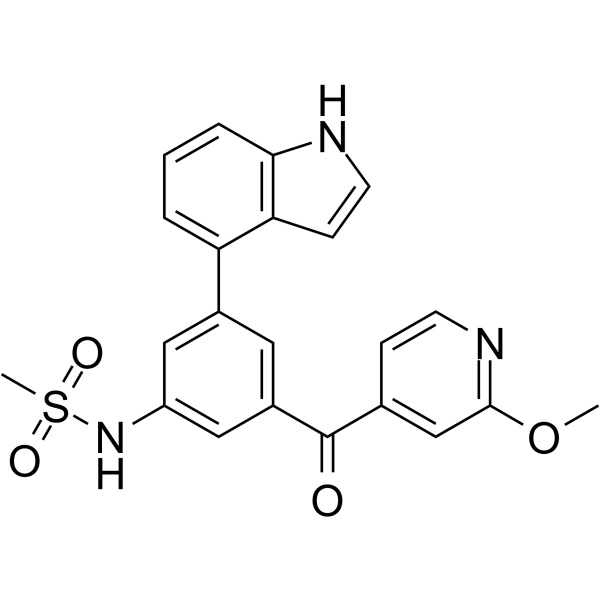
| SMILES | COC1=NC=CC(=C1)C(=O)C2=CC(=CC(=C2)C3=C4C=CNC4=CC=C3)NS(=O)(=O)C |
| InChi Key | YUVDELGTFILMBB-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C22H19N3O4S/c1-29-21-13-14(6-8-24-21)22(26)16-10-15(11-17(12-16)25-30(2,27)28)18-4-3-5-20-19(18)7-9-23-20/h3-13,23,25H,1-2H3 |
| Chemical Name | N-[3-(1H-indol-4-yl)-5-(2-methoxypyridine-4-carbonyl)phenyl]methanesulfonamide |
| Synonyms | LP261; LP-261; LP 261 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | tubulin polymerization (EC50 = 3.2 μM) |
| ln Vitro |
LP-261 shows a range of activity from 0.01μM to 0.38μM across the tested cell lines, and demonstrates potent G2/M block activity in multiple of them. The IC50 values for MCF-7, H522, Jurkat, SW-620, BXPC-3, and PC-3 are 0.01μM, 0.01μM, 0.02μM, 0.05μM, 0.05μM, and 0.07μM, respectively[1]. LP-261 demonstrates low micromolar potency; its EC50 value is 5.0 μM in the tubulin polymerization assay[1]. In a [3H]colchicine competition binding assay, LP-261 can rival colchicine for binding to tubulin. Its EC50 (3.2 μM) indicates that it can inhibit binding with a potency comparable to that of colchicine, and it can exhibit a 79% inhibition at a concentration of 30 μM[1]. |
| ln Vivo | LP-261 (oral gavage; 4 mg/kg; single dose) exhibits a moderate rate of elimination in rats, with a terminal half-life of 1.4 hours (0.2 hours) indicating rapid oral adsorption (Tmax=2.0 h), and a volume of distribution (Vss) of 1.25 L/kg[1]. LP-261 (oral gavage; 15 or 50 mg/kg; twice daily; 28 days) at 50 mg/kg causes an approximate tumor volume of 130 mm3, which is 96% less than the mean tumor volume in the vehicle treated group, which was 3769 mm3. In this mouse model, LP-261 at 15 mg/kg results in a 41% inhibition after 28 days[1]. |
| Animal Protocol |
Human tumor xenograft model (Injected with NCI-H522 human non-small-cell) in NCr-nu mice[1] 15 or 50 mg/kg Oral gavage; 15 or 50 mg/kg; twice daily; 28 days |
| References |
[1]. Synthesis and pharmacological evaluation of N-(3-(1H-indol-4-yl)-5-(2-methoxyisonicotinoyl)phenyl)methanesulfonamide (LP-261), a potent antimitotic agent. J Med Chem. 2011 Jan 13;54(1):179-200 . |
Solubility Data
| Solubility (In Vitro) | DMSO: ~33.3 mg/mL (~79.1 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.93 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.93 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3726 mL | 11.8632 mL | 23.7265 mL | |
| 5 mM | 0.4745 mL | 2.3726 mL | 4.7453 mL | |
| 10 mM | 0.2373 mL | 1.1863 mL | 2.3726 mL |