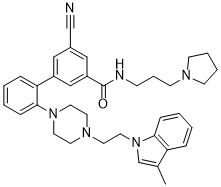
LLY-507 (LLY507) is a novel, potent, cell-permeable/active and selective inhibitor of protein-lysine methyltransferase SMYD2 (SET And MYND Domain Containing 2) with potential anticancer activity. It inhibits SMYD2 with an IC50 of 15 nM. SMYD2 is a lysine methyltransferase that catalyzes the monomethylation of several protein substrates including p53. SMYD2 is overexpressed in a significant percentage of esophageal squamous primary carcinomas, and that overexpression correlates with poor patient survival. LLY-507 is >100-fold selective for SMYD2 over a broad range of methyltransferase and non-methyltransferase targets. LLY-507 is active in cells as measured by reduction of SMYD2-induced monomethylation of p53 Lys(370) at submicromolar concentrations. LLY-507 inhibited the proliferation of several esophageal, liver, and breast cancer cell lines in a dose-dependent manner. LLY-507 serves as a valuable chemical probe to aid in the dissection of SMYD2 function in cancer and other biological processes.
Physicochemical Properties
| Molecular Formula | C36H42N6O |
| Molecular Weight | 574.758287906647 |
| Exact Mass | 574.341 |
| CAS # | 1793053-37-8 |
| PubChem CID | 91623361 |
| Appearance | White to off-white solid powder |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 790.4±70.0 °C at 760 mmHg |
| Flash Point | 431.8±35.7 °C |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.646 |
| LogP | 7.27 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 10 |
| Heavy Atom Count | 43 |
| Complexity | 935 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | PNYRDVBFYVDJJI-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C36H42N6O/c1-28-27-42(34-11-4-2-9-32(28)34)22-19-40-17-20-41(21-18-40)35-12-5-3-10-33(35)30-23-29(26-37)24-31(25-30)36(43)38-13-8-16-39-14-6-7-15-39/h2-5,9-12,23-25,27H,6-8,13-22H2,1H3,(H,38,43) |
| Chemical Name | 3-cyano-5-[2-[4-[2-(3-methylindol-1-yl)ethyl]piperazin-1-yl]phenyl]-N-(3-pyrrolidin-1-ylpropyl)benzamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | LLY-507 targets over twenty-one other methyltransferases, including SMYD3 in addition to SMYD2[1]. LLY-507 supports SMYD2's inhibitory action by binding to its substrate channel [1]. SMYD2 LLY-507 (0.03 – 20 μM; 28 hours) inhibits p53 Lys370 methylation mediated by SMYD2 in U2OS cells (IC50 0.6 μM[1]); enzyme methylation of H4 peptide (IC50 31 nM[1]). The growth of several ESCC, HCC, and breast cancer cell lines is inhibited by LLY-507 (0–20 μM; 3-7) [1]. |
| Cell Assay |
Cell proliferation assay [1] Cell Types: ESCC, HCC, breast cancer cell line Tested Concentrations:0-20 μM Incubation Duration: 3 days, 7 days Experimental Results: Inhibit tumor cell proliferation. Western Blot Analysis[1] Cell Types: HEK293 Cell Tested Concentrations: 0.03 μM, 0.07 μM, 0.15 μM, 0.3 μM, 0.6 μM, 1.25 μM, 2.5 μM Incubation Duration: 28 hrs (hours) Experimental Results: Inhibition of SMYD2-mediated p53 Lys370 alpha in cells base. |
| References |
[1]. LLY-507, a Cell-active, Potent, and Selective Inhibitor of Protein-lysine Methyltransferase SMYD2. J Biol Chem. 2015 May 29;290(22):13641-13653. |
| Additional Infomation | LLY-507 is a secondary carboxamide resulting from the formal condensation of the carboxy group of 5-cyano-2'-{4-[2-(3-methyl-1H-indol-1-yl)ethyl]piperazin-1-yl}[biphenyl]-3-carboxylic acid with the amino group of 3-(pyrrolidin-1-yl)propan-1-amine. It is a potent and selective inhibitor of SMYD2 and inhibits the ability of SMYD2 to methylate p53. It serves as a valuable chemical probe to aid in the dissection of SMYD2 function in cancer and other biological processes. It has a role as an EC 2.1.1.354 ([histone H3]-lysine(4) N-trimethyltransferase) inhibitor. It is a methylindole, a N-alkylpiperazine, a N-arylpiperazine, a nitrile, a member of benzamides, a secondary carboxamide and a N-alkylpyrrolidine. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~15 mg/mL (~26.10 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (4.35 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.35 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7399 mL | 8.6993 mL | 17.3986 mL | |
| 5 mM | 0.3480 mL | 1.7399 mL | 3.4797 mL | |
| 10 mM | 0.1740 mL | 0.8699 mL | 1.7399 mL |