LF3 is a specific antagonist of the β-Catenin/TCF4 interaction with antitumor activity; it acts by disrupting the interaction between β-catenin and TCF4 with an IC50 of 1.65 μM. LF3 does not cause cell death or interfere with cadherin-mediated cell-cell adhesion. The self-renewal capacity of cancer stem cells is blocked by LF3 in concentration-dependent manners, as examined by sphere formation of colon and head and neck cancer stem cells under nonadherent conditions. LF3 inhibits Wnt/β-catenin signaling, but does not interfere with E-cadherin/β-catenin-mediated cell-cell adhesion.
Physicochemical Properties
| Molecular Formula | C20H24N4O2S2 | |
| Molecular Weight | 416.56 | |
| Exact Mass | 416.134 | |
| Elemental Analysis | C, 57.67; H, 5.81; N, 13.45; O, 7.68; S, 15.39 | |
| CAS # | 664969-54-4 | |
| Related CAS # |
|
|
| PubChem CID | 1213452 | |
| Appearance | White to off-white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 609.6±65.0 °C at 760 mmHg | |
| Flash Point | 322.5±34.3 °C | |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C | |
| Index of Refraction | 1.680 | |
| LogP | 1.97 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 28 | |
| Complexity | 625 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | S=C(N([H])C1C([H])=C([H])C(=C([H])C=1[H])S(N([H])[H])(=O)=O)N1C([H])([H])C([H])([H])N(C([H])([H])/C(/[H])=C(\[H])/C2C([H])=C([H])C([H])=C([H])C=2[H])C([H])([H])C1([H])[H] |
|
| InChi Key | ZUQIFHLBPBLRRM-QPJJXVBHSA-N | |
| InChi Code | InChI=1S/C20H24N4O2S2/c21-28(25,26)19-10-8-18(9-11-19)22-20(27)24-15-13-23(14-16-24)12-4-7-17-5-2-1-3-6-17/h1-11H,12-16H2,(H,22,27)(H2,21,25,26)/b7-4+ | |
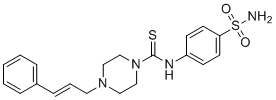
| Chemical Name | 4-[(E)-3-phenylprop-2-enyl]-N-(4-sulfamoylphenyl)piperazine-1-carbothioamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-Catenin/TCF4 (IC50 = 1.65 μM by AlphaScreen; and 1.82 μM by ELISA)[1] | ||
| ln Vitro | In cells containing exogenous reporters and colon cancer cells with high endogenous Wnt activity, LF3 suppresses Wnt/β-catenin signals. Moreover, LF3 inhibits the overexpression of Wnt target genes, high cell motility, and cell-cycle progression in cancer cells that are connected to Wnt signaling. LF3 does not, however, impair cadherin-mediated cell-cell adhesion or result in cell death. Surprisingly, LF3 inhibits cancer stem cells' ability to self-renew in ways that depend on concentration[1]. | ||
| ln Vivo | LF3 inhibits tumor growth and promotes differentiation in a colon cancer mouse xenograft model. When LF3 at 50 mg/kg is administered to mice with GFPhigh cells, tumor growth is markedly inhibited. The normal histology of mice's guts is not affected by LF3 treatment[1]. | ||
| Enzyme Assay | Analogues of LF3 were dissolved in DMSO to a concentration of 50 mmol/L. In the AlphaScreens, purified GST-hβ-catenin was first incubated with nickel chelate acceptor beads and His-hTCF4 incubated with glutathione donor beads (Perkinelmer) in PBS. They were then combined into 384-well AlphaPlates in the presence of 20 μmol/L test compounds. Titration was used to determine the concentration of purified proteins used in the AlphaScreens, yielding signals in linear ranges. After 1-hour incubation, protein–protein interactions induced luminescence, as measured by an EnVision multilabel plate reader[1]. | ||
| Cell Assay | Twenty-four–well cell culture plates were precoated with 250 μL polyhema (12 mg/mL in 95% ethanol; Sigma) to promote the growth of nonattached spheres. SW480 reporter cells and mouse salivary gland CSCs were trypsinized and seeded as single cells into the sphere culture medium (F12:DMEM 1:1, 1× B-27 supplement, 20 ng/mL EGF, 20 ng/mL FGF, 0.5% methylcellulose) with or without treatment. After 10 days, spheres were counted under a phase contrast microscope, and pictures were taken by AF6000 and DFC350FX[1]. | ||
| Animal Protocol |
|
||
| References |
[1]. A Small-Molecule Antagonist of the β-Catenin/TCF4 Interaction Blocks the Self-Renewal of Cancer Stem Cells and Suppresses Tumorigenesis. Cancer Res. 2016 Feb 15;76(4):891-901. |
||
| Additional Infomation | Wnt/β-catenin signaling is a highly conserved pathway essential for embryogenesis and tissue homeostasis. However, deregulation of this pathway can initiate and promote human malignancies, especially of the colon and head and neck. Therefore, Wnt/β-catenin signaling represents an attractive target for cancer therapy. We performed high-throughput screening using AlphaScreen and ELISA techniques to identify small molecules that disrupt the critical interaction between β-catenin and the transcription factor TCF4 required for signal transduction. We found that compound LF3, a 4-thioureido-benzenesulfonamide derivative, robustly inhibited this interaction. Biochemical assays revealed clues that the core structure of LF3 was essential for inhibition. LF3 inhibited Wnt/β-catenin signals in cells with exogenous reporters and in colon cancer cells with endogenously high Wnt activity. LF3 also suppressed features of cancer cells related to Wnt signaling, including high cell motility, cell-cycle progression, and the overexpression of Wnt target genes. However, LF3 did not cause cell death or interfere with cadherin-mediated cell-cell adhesion. Remarkably, the self-renewal capacity of cancer stem cells was blocked by LF3 in concentration-dependent manners, as examined by sphere formation of colon and head and neck cancer stem cells under nonadherent conditions. Finally, LF3 reduced tumor growth and induced differentiation in a mouse xenograft model of colon cancer. Collectively, our results strongly suggest that LF3 is a specific inhibitor of canonical Wnt signaling with anticancer activity that warrants further development for preclinical and clinical studies as a novel cancer therapy.[1] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.00 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (4.99 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (4.99 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4006 mL | 12.0031 mL | 24.0061 mL | |
| 5 mM | 0.4801 mL | 2.4006 mL | 4.8012 mL | |
| 10 mM | 0.2401 mL | 1.2003 mL | 2.4006 mL |