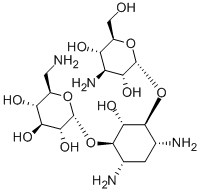
Kanamycin (Kanamycin A; Kantrex; Kanamycine) is a novel and potent aminoglycoside bacteriocidal antibiotic effective against gram-positive and negative bacteria and mycoplasma. It has been used for the prevention and treatment of chicken pullorum, colibacillosis, enteritis and other diseases, and has a significant effect on systemic sepsis, respiratory tract infection, peritonitis, etc. caused by drug-resistant bacteria.Aminoglycosides like kanamycin irreversibly bind to specific 30S-subunit proteins and 16S rRNA. Specifically Kanamycin binds to four nucleotides of 16S rRNA and a single amino acid of protein S12.
Physicochemical Properties
| Molecular Formula | C18H36N4O11 |
| Molecular Weight | 484.503 |
| Exact Mass | 484.238 |
| Elemental Analysis | C, 44.62; H, 7.49; N, 11.56; O, 36.32 |
| CAS # | 59-01-8 |
| Related CAS # | Kanamycin sulfate;25389-94-0 |
| PubChem CID | 6032 |
| Appearance | White to off-white solid powder |
| Density | 1.6±0.1 g/cm3 |
| Boiling Point | 809.5±65.0 °C at 760 mmHg |
| Flash Point | 443.4±34.3 °C |
| Vapour Pressure | 0.0±6.5 mmHg at 25°C |
| Index of Refraction | 1.670 |
| LogP | -2.58 |
| Hydrogen Bond Donor Count | 11 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 33 |
| Complexity | 638 |
| Defined Atom Stereocenter Count | 15 |
| SMILES | C1[C@H]([C@@H]([C@H]([C@@H]([C@H]1N)OC2[C@@H]([C@H]([C@@H]([C@@H](CN)O2)O)O)O)O)O[C@@H]3[C@@H]([C@H]([C@@H]([C@@H](CO)O3)O)N)O)N |
| InChi Key | SBUJHOSQTJFQJX-NOAMYHISSA-N |
| InChi Code | InChI=1S/C18H36N4O11/c19-2-6-10(25)12(27)13(28)18(30-6)33-16-5(21)1-4(20)15(14(16)29)32-17-11(26)8(22)9(24)7(3-23)31-17/h4-18,23-29H,1-3,19-22H2/t4-,5+,6-,7-,8+,9-,10-,11-,12+,13-,14-,15+,16-,17-,18-/m1/s1 |
| Chemical Name | (2R,3S,4S,5R,6R)-2-(aminomethyl)-6-(((1R,2R,3S,4R,6S)-4,6-diamino-3-(((2S,3R,4S,5S,6R)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-2-hydroxycyclohexyl)oxy)tetrahydro-2H-pyran-3,4,5-triol |
| Synonyms | Kanamycin A; Kanamycin free base; Kanamycin A; Kantrex; Kanamycine. |
| HS Tariff Code | 2941.90.1010 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Aminoglycoside |
| ln Vitro | When applied to different strains of mycobacteria in vitro, kanamycin (0.1-100 μg/mL; 2 weeks) shows good antibacterial activity (MIC=1-5 μg/mL)[1]. |
| ln Vivo | In the lung and spleen of mice, kanamycin (2, 4 mg/kg; s.c.; once daily, six times a week for three weeks) inhibits the growth of bovine tubercle bacilli[1]. In addition to increasing the survival rate of mice, kanamycin (1.25, 5 mg/kg; s.c.; single (at 3 h after infection)) inhibits the multiplication of K. pneumonia DT-S in the lung, trachea, and blood of mice and in proportion to the dose administration[2]. |
| Cell Assay |
Cell Line: mycobacteria H2 and H37RvR-PAS, BCG, Kirchbergand, Ravenel, and H37Rv. Concentration: 0.1-100 μg/mL Incubation Time: 2 weeks Result: demonstrated strong antibacterial activity against multiple mycobacterium strains (H37Rv, H2, H37RvR-PAS, Ravenel, and BCG), with MICs of 1 μg/mL and 5 μg/mL for the Kirchbergand strain, respectively. |
| Animal Protocol |
Animal Model: Inbred strain normal mice (14-16 g; bovine tubercle bacilli (Ravenel strain) infected model). Dosage: 2, 4 mg/kg Administration: Subcutaneous injection: once a day for three weeks, six times a week. Result: had a noticeable impact on preventing the spread of tuberculosis in vivo, particularly in mice's lungs. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Kanamycin is rapidly absorbed after intramuscular injection and peak serum levels are generally reached within approximately one hour. Poor oral and topical absorption except with severe skin damage. In one study in premature infants, peak plasma kanamycin concentrations averaging 17.5 ug/mL were attained within 1 hour following IM administration of a single kanamycin dose of 6.3-8.5 mg/kg; plasma concentrations of the drug averaged 5.8 ug/mL at 12 hours. In neonates 1-7 days of age, peak serum concentrations 30 minutes after a 7.5-mg/kg or 10-mg/kg IM dose were 21.8 or 26.8 ug/mL, respectively. When these doses were given by IV infusion over 20 minutes, serum concentrations at 30 minutes were 21.4 or 29.3 ug/mL, respectively. Kanamycin is poorly absorbed from the GI tract. In one study, intraperitoneal instillation of 500 mg of kanamycin diluted in 20 mL of 0.9% sodium chloride resulted in peak plasma kanamycin concentrations of 19 ug/mL within 15 minutes. Kanamycin is rapidly absorbed after IM injection. Following IM administration of a single 7.5-mg/kg dose of kanamycin in adults with normal renal function, peak plasma kanamycin concentrations are attained within approximately 1 hour and average 22 ug/mL;1 at 8 hours after the dose, plasma concentrations of the drug average 3.2 ug/mL.1 Similar plasma concentrations of kanamycin are attained when the same dose is administered by IV infusion over 1 hour. For more Absorption, Distribution and Excretion (Complete) data for Kanamycin A (11 total), please visit the HSDB record page. Metabolism / Metabolites Aminoglycosides are not metabolized and are excreted unchanged in the urine primarily by glomerular filtration. /Aminoglycosides/ Biological Half-Life 2.5 hours The plasma elimination half-life of kanamycin is 2-4 hours in adults with normal renal function, but may be prolonged in geriatric patients. In neonates 1-7 days of age, half-life averaged 4.3-5.1 hours. In premature infants, the elimination half-life of kanamycin reportedly averages 9 hours. In patients with severe burns, plasma half-life and plasma concentrations of the drug may be decreased. Plasma concentrations are higher and the elimination half-life of kanamycin is prolonged in patients with renal impairment. In those with severe renal impairment, the plasma half-life of kanamycin may range from 27-80 hours. |
| Toxicity/Toxicokinetics |
Toxicity Summary IDENTIFICATION AND USE: Kanamycin A is aminoglycoside anti-bacterial agent. Kanamycin injection is indicated in the short-term treatment of serious infections caused by susceptible strains of the designated microorganisms. Kanamycin may be considered as initial therapy in the treatment of infections where one or more of the following are the known or suspected pathogens: E. coli, Proteus species (both indole-positive and indole-negative), Enterobacter aerogenes, Klebsiella pneumoniae, Serratia marcescens, Acinetobacter species. HUMAN EXPOSURE AND TOXICITY: Toxic effects of kanamycin on the eighth cranial nerve can result in partially reversible or irreversible bilateral loss of hearing, loss of balance, or both. Tinnitus or vertigo may or may not be experienced. Cochlear damage is usually manifested initially by small changes in audiometric test results at the high frequencies and may not be associated with subjective hearing loss. Vestibular dysfunction is usually manifested by nystagmus, vertigo, nausea, vomiting, or acute Meniere's syndrome. Serious sensitivity reactions, such as anaphylaxis and dermatologic reactions including exfoliative dermatitis, toxic epidermal necrolysis, erythema multiforme, angioedema, and Stevens-Johnson syndrome, have been reported rarely in patients receiving aminoglycosides; fatalities have occurred rarely. Cross-sensitivity occurs among the aminoglycosides. ANIMAL STUDIES: Intravitreal injection in rabbits and owl monkeys was tolerated at a dose of 0.5 mg, but 1.5 to 6.0 mg produced cataracts in rabbits. Dosages of 200 mg/kg/day in pregnant rats and pregnant guinea pigs led to hearing impairment in the offspring. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation If kanamycin is required by the mother, it is not a reason to discontinue breastfeeding. Kanamycin is poorly excreted into breastmilk. Newborn infants apparently absorb small amounts of other aminoglycosides, but serum levels with typical three times daily dosages are far below those attained when treating newborn infections and systemic effects of kanamycin are unlikely. Older infants would be expected to absorb even less kanamycin. Because there is little variability in the milk kanamycin levels during multiple daily dose regimens, timing breastfeeding with respect to the dose is of little or no benefit in reducing infant exposure. Data are not available with single daily dose regimens. Monitor the infant for possible effects on the gastrointestinal flora, such as diarrhea, candidiasis (e.g., thrush, diaper rash) or rarely, blood in the stool indicating possible antibiotic-associated colitis. ◉ Effects in Breastfed Infants Kanamycin was used as part of a six-drug regimen to treat a pregnant woman with multidrug-resistant tuberculosis during the first trimester of pregnancy and postpartum. The infant was breastfed (extent and duration not stated). At age 1.8 years, the child had failure to thrive, possibly due to tuberculosis contracted after birth, but was otherwise developing normally. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Interactions Concomitant and/or sequential use of an aminoglycoside and other systemic, oral, or topical drugs that have neurotoxic, ototoxic, or nephrotoxic effects (e.g., other aminoglycosides, acyclovir, amphotericin B, bacitracin, capreomycin, certain cephalosporins, colistin, cisplatin, methoxyflurane, polymyxin B, vancomycin) may result in additive toxicity and should be avoided, if possible. /Aminoglycosides/ Because of the possibility of an increased risk of ototoxicity due to additive effects or altered serum and tissue aminoglycoside concentrations, aminoglycosides should not be given concomitantly with potent diuretics such as ethacrynic acid, furosemide, urea, or mannitol. It has been suggested that concomitant use of certain anti-emetics that suppress nausea and vomiting of vestibular origin and vertigo (e.g., dimenhydrinate, meclizine) may mask symptoms of aminoglycoside-associated vestibular ototoxicity. /Aminoglycosides/ Concurrent use of an aminoglycoside with general anesthetics or neuromuscular blocking agents (e.g., succinylcholine, rocuronium, tubocurarine) may potentiate neuromuscular blockade and cause respiratory paralysis. ... Aminoglycosides should be used with caution in patients receiving anesthetics or neuromuscular blocking agents, and patients should be closely observed for signs of respiratory depression. /Aminoglycosides/ In vitro studies indicate that the antibacterial activity of aminoglycosides and beta-lactam antibiotics may be additive or synergistic against some organisms including Enterobacteriaceae, Pseudomonas aeruginosa, enterococci, and viridans streptococci. The synergistic effect of aminoglycosides and beta-lactams is used to therapeutic advantage, especially in the treatment of infections caused by enterococci or Ps. aeruginosa. Although the exact mechanism of this synergistic effect has not been determined, it appears that by inhibiting bacterial cell-wall synthesis the penicillin allows more effective ingress of the aminoglycoside to the ribosomal binding site. Synergism between aminoglycosides and extended-spectrum penicillins generally is unpredictable and antagonism has been reported rarely in vitro when these penicillins were used in conjunction with amikacin, gentamicin, or tobramycin. Therefore, some clinicians suggest that when concomitant therapy is indicated it may be advisable to use appropriate in vitro studies to demonstrate synergism against the isolated organism. Concomitant administration of an extended-spectrum penicillin and an aminoglycoside has resulted in decreased serum aminoglycoside concentrations and elimination half life, especially in patients with renal impairment. Therefore, serum aminoglycoside concentrations should be monitored in patients receiving concomitant therapy, especially when very high doses of an extended-spectrum penicillin are used or when the patient has impaired renal function. /Aminoglycosides/ For more Interactions (Complete) data for Kanamycin A (10 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat iv 437 mg/kg LD50 Rabbit iv 150 mg/kg LD50 Mouse iv 115 mg/kg LD50 Mouse sc 1350 mg/kg For more Non-Human Toxicity Values (Complete) data for Kanamycin A (7 total), please visit the HSDB record page. |
| References |
[1]. Studies on kanamycin, a new antibiotic against tubercle bacilli. I. Effect on virulent tubercle bacilli in vitro and in mice. J Antibiot (Tokyo). 1957 Nov;10(6):233-5. [2]. Experimental respiratory tract infection with Klebsiella pneumoniae DT-S in mice: chemotherapy with kanamycin. Antimicrob Agents Chemother. 1980 Mar;17(3):494-505. [3]. Interaction of kanamycin and related antibiotics with the large subunit of ribosomes and the inhibition of translocation. Biochem Biophys Res Commun. 1978 Sep 29;84(2):358-65. [4]. Mechanism of inhibition of translocation by kanamycin and viomycin: a comparative study with fusidic acid. Biochem Biophys Res Commun. 1980 Jan 29;92(2):647-54. |
| Additional Infomation |
Kanamycin A is a member of kanamycins. It has a role as a bacterial metabolite. It is a conjugate base of a kanamycin A(4+). Kanamycin (also known as kanamycin A) is an aminoglycoside bacteriocidal antibiotic, available in oral, intravenous, and intramuscular forms, and used to treat a wide variety of infections. Kanamycin is isolated from the bacterium Streptomyces kanamyceticus and its most commonly used form is kanamycin sulfate. Kanamycin has been reported in Hohenbuehelia grisea, Streptomyces, and other organisms with data available. Kanamycin is an aminoglycoside antibiotic with antimicrobial property. Amikacin irreversibly binds to the bacterial 30S ribosomal subunit, specifically in contact with 16S rRNA and S12 protein within the 30S subunit. This leads to interference with translational initiation complex and, misreading of mRNA, thereby hampering protein synthesis and resulting in bactericidal effect. This agent is usually used for treatment of E. coli, Proteus species (both indole-positive and indole-negative), E. aerogenes, K. pneumoniae, S. marcescens, and Acinetobacter species. Kanamycin A is the major component of the kanamycin complex, an aminoglycoside antibiotic isolated from Streptomyces kanamyceticus, with antibacterial activity. Antibiotic complex produced by Streptomyces kanamyceticus from Japanese soil. Comprises 3 components: kanamycin A, the major component, and kanamycins B and C, the minor components. Drug Indication For treatment of infections where one or more of the following are the known or suspected pathogens: E. coli, Proteus species (both indole-positive and indole-negative), E. aerogenes, K. pneumoniae, S. marcescens, and Acinetobacter species. Mechanism of Action Aminoglycosides like kanamycin "irreversibly" bind to specific 30S-subunit proteins and 16S rRNA. Specifically Kanamycin binds to four nucleotides of 16S rRNA and a single amino acid of protein S12. This interferes with decoding site in the vicinity of nucleotide 1400 in 16S rRNA of 30S subunit. This region interacts with the wobble base in the anticodon of tRNA. This leads to interference with the initiation complex, misreading of mRNA so incorrect amino acids are inserted into the polypeptide leading to nonfunctional or toxic peptides and the breakup of polysomes into nonfunctional monosomes. Kanamycin, an aminoglycoside, acts by inhibiting the synthesis of protein in susceptible microorganisms. It is bactericidal in vitro against Gram-negative bacteria and certain Gram-positive bacteria. Aminoglycosides are usually bactericidal in action. Although the exact mechanism of action has not been fully elucidated, the drugs appear to inhibit protein synthesis in susceptible bacteria by irreversibly binding to 30S ribosomal subunits. /Aminoglycosides/ ... Aminoglycosides are aminocyclitols that kill bacteria by inhibiting protein synthesis as they bind to the 16S rRNA and by disrupting the integrity of bacterial cell membrane. Aminoglycoside resistance mechanisms include: (a) the deactivation of aminoglycosides by N-acetylation, adenylylation or O-phosphorylation, (b) the reduction of the intracellular concentration of aminoglycosides by changes in outer membrane permeability, decreased inner membrane transport, active efflux, and drug trapping, (c) the alteration of the 30S ribosomal subunit target by mutation, and (d) methylation of the aminoglycoside binding site. ... /Aminoglycosides/ Therapeutic Uses Anti-Bacterial Agents; Protein Synthesis Inhibitors /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Kanamycin A is included in the database. Kanamycin injection is indicated in the short-term treatment of serious infections caused by susceptible strains of the designated microorganisms: /Escherichia coli, Proteus species (both indole-positive and indole-negative), Enterobacter aerogenes, Klebsiella pneumoniae, Serratia marcescens, Acinetobacter species/. Bacteriological studies to identify the causative organisms and to determine their susceptibility to kanamycin should be performed. Therapy may be instituted prior to obtaining the results of susceptibility testing. /Included in US product label/ Kanamycin may be considered as initial therapy in the treatment of infections where one or more of the following are the known or suspected pathogens: E. coli, Proteus species (both indole-positive and indole-negative), Enterobacter aerogenes, Klebsiella pneumoniae, Serratia marcescens, Acinetobacter species. /Included in US product label/ For more Therapeutic Uses (Complete) data for Kanamycin A (9 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ Patients treated with aminoglycosides by any route should be under close clinical observation because of the potential toxicity associated with their use. As with other aminoglycosides, the major toxic effects of kanamycin are its action on the auditory and vestibular branches of the eighth nerve and the renal tubules. Neurotoxicity is manifested by bilateral auditory toxicity which often is permanent and, sometimes, by vestibular ototoxicity. Loss of high frequency perception usually occurs before there is noticeable clinical hearing loss and can be detected by audiometric testing. There may not be clinical symptoms to warn of developing cochlear damage. Vertigo may occur and may be evidence of vestibular injury. Other manifestations of neurotoxicity may include numbness, skin tingling, muscle twitching, and convulsions. The risk of hearing loss increases with the degree of exposure to either high peak or high trough serum concentrations and continues to progress after drug withdrawal. Renal impairment may be characterized by decreased creatinine clearance, the presence of cells or casts, oliguria, proteinuria, decreased urine specific gravity, or evidence of increasing nitrogen retention (increasing BUN, NPN, or serum creatinine). The risks of severe ototoxic and nephrotoxic reactions are sharply increased in patients with impaired renal function and in those with normal renal function who receive high doses or prolonged therapy. Renal and eighth nerve function should be closely monitored, especially in patients with known or suspected reduced renal function at the onset of therapy, and also in those whose renal function is initially normal but who develop signs of renal dysfunction during therapy. Serum concentrations of parenterally administered aminoglycosides should be monitored when feasible to assure adequate levels and to avoid potentially toxic levels. Urine should be examined for decreased specific gravity, increased excretion of protein, and the presence of cells or casts. Blood urea nitrogen, serum creatinine, or creatinine clearance should be measured periodically. Serial audiograms should be obtained when feasible in patients old enough to be tested; particularly high risk patients. Evidence of ototoxicity (dizziness, vertigo, tinnitus, roaring in the ears, and hearing loss) or nephrotoxicity requires dosage adjustment or discontinuance of the drug. Neuromuscular blockade with respiratory paralysis may occur when kanamycin is instilled intraperitoneally concomitantly with anesthesia and muscle-relaxing drugs. Neuromuscular blockade has been reported following parenteral injection and the oral use of aminoglycosides. The possibility of the occurrence of neuromuscular blockade and respiratory paralysis should be considered if aminoglycosides are administered by any route, especially in patients receiving anesthetics, neuromuscular-blocking agents such as tubocurarine, succinylcholine, decamethonium, or in patients receiving massive transfusions of citrate-anticoagulated blood. If blockage occurs, calcium salts may reduce these phenomena but mechanical respiratory assistance may be necessary. The concurrent and/or sequential systemic, oral, or topical use of kanamycin and other potentially nephrotoxic, and/or neurotoxic drugs, particularly polymyxin B, bacitracin, colistin, amphotericin B, cisplatin, vancomycin, and all other aminoglycosides (including paromomycin) should be avoided because the toxicity may be additive. Other factors which may increase patient risk of toxicity are advanced age and dehydration. Kanamycin should not be given concurrently with potent diuretics (ethacrynic acid, furosemide, meralluride sodium, sodium mercaptomerin, or mannitol). Some diuretics themselves cause ototoxicity, and intravenously administered diuretics may enhance aminoglycoside toxicity by altering antibiotic concentrations in serum and tissue. Kanamycin has the potential to induce auditory and sometimes vestibular toxicity, renal toxicity, and neuromuscular blockade. The risks are higher for patients with a present or past history of renal impairment (especially if hemodialysis is required), for those receiving concomitant or sequential treatment with other ototoxic or nephrotoxic drugs or rapid acting diuretic agents given intravenously (ethacrynic acid, furosemide, and mannitol), and for patients treated for longer periods and/or with higher doses than recommended. Some local irritation or pain may follow the intramuscular injection of kanamycin. Other adverse reactions of the drug reported on rare occasions are skin rash, drug fever, headache, paresthesia, nausea, vomiting, and diarrhea. The "malabsorption syndrome" characterized by an increase in fecal fat, decrease in serum carotene, and fall in xylose absorption, reportedly has occurred with prolonged therapy. Albuminuria, presence of red and white cells, and granular casts; azotemia and oliguria have been reported. Renal function changes are usually reversible when the drug /kanamycin/ is discontinued. Renal impairment may be characterized by a rise in serum creatinine and may be accompanied by oliguria, presence of casts, cells, and protein in the urine, by rising levels of BUN or by decrease in creatinine clearance. For more Drug Warnings (Complete) data for Kanamycin A (32 total), please visit the HSDB record page. Pharmacodynamics Kanamycin is an aminoglycoside antibiotic. Aminoglycosides work by binding to the bacterial 30S ribosomal subunit, causing misreading of t-RNA, leaving the bacterium unable to synthesize proteins vital to its growth. Aminoglycosides are useful primarily in infections involving aerobic, Gram-negative bacteria, such as Pseudomonas, Acinetobacter, and Enterobacter. In addition, some mycobacteria, including the bacteria that cause tuberculosis, are susceptible to aminoglycosides. Infections caused by Gram-positive bacteria can also be treated with aminoglycosides, but other types of antibiotics are more potent and less damaging to the host. In the past the aminoglycosides have been used in conjunction with penicillin-related antibiotics in streptococcal infections for their synergistic effects, particularly in endocarditis. Aminoglycosides are mostly ineffective against anaerobic bacteria, fungi and viruses. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0640 mL | 10.3199 mL | 20.6398 mL | |
| 5 mM | 0.4128 mL | 2.0640 mL | 4.1280 mL | |
| 10 mM | 0.2064 mL | 1.0320 mL | 2.0640 mL |