Physicochemical Properties
| Molecular Formula | C45H51F3N8O7 |
| Molecular Weight | 872.93 |
| Exact Mass | 872.383 |
| CAS # | 2573298-13-0 |
| PubChem CID | 165437238 |
| Appearance | Light yellow to yellow solid powder |
| LogP | 5 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 14 |
| Heavy Atom Count | 63 |
| Complexity | 1690 |
| Defined Atom Stereocenter Count | 0 |
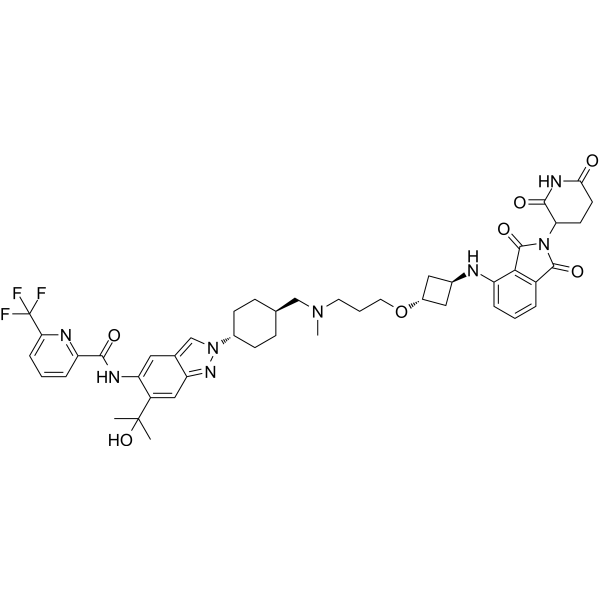
| SMILES | N([C@@H]1C[C@H](C1)OCCCN(C)C[C@@H]1CC[C@@H](N2N=C3C=C(C(=CC3=C2)NC(C2=CC=CC(=N2)C(F)(F)F)=O)C(O)(C)C)CC1)C1C=CC=C2C(N(C3CCC(NC3=O)=O)C(C2=1)=O)=O |
| InChi Key | CKFOAVDINFHZIR-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C45H51F3N8O7/c1-44(2,62)31-22-34-26(19-35(31)51-40(58)33-9-5-10-37(50-33)45(46,47)48)24-55(53-34)28-13-11-25(12-14-28)23-54(3)17-6-18-63-29-20-27(21-29)49-32-8-4-7-30-39(32)43(61)56(42(30)60)36-15-16-38(57)52-41(36)59/h4-5,7-10,19,22,24-25,27-29,36,49,62H,6,11-18,20-21,23H2,1-3H3,(H,51,58)(H,52,57,59) |
| Chemical Name | N-[2-[4-[[3-[3-[[2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-4-yl]amino]cyclobutyl]oxypropyl-methylamino]methyl]cyclohexyl]-6-(2-hydroxypropan-2-yl)indazol-5-yl]-6-(trifluoromethyl)pyridine-2-carboxamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | DC50: 4 nM (IRAK4), 5 nM (Ikaros)[1] |
| ln Vitro | In whole blood monocytes and lymphocytes, KTX-582 (compound I-41) degrades IRAK4 with IC50s of less than 0.05 μM[4]. In human whole blood LPS TNFα, KTX-582 inhibits IRAK4 with an IC50 of 0.05~1 μM[4]. |
| References |
[1]. Matthew Weiss. Discovery and characterization of IRAKIMiDs: degraders targeting both IRAK4 and IMiD substrates for oncology indications. Northeastern Section, ACS (NESACS). [2]. Jennifer K. Lue, MD . Targeting MYD88-Mutant DLBCL with IRAKIMiDs: A Comparison to IRAK4 Kinase Inhibition and Evaluation of Synergy with Rational Combinations. American Society of Hematology ASH Annual Meeting. [3]. Vogelmann A, Robaa D, Sippl W, Jung M. Proteolysis targeting chimeras (PROTACs) for epigenetics research. Curr Opin Chem Biol. 2020 Aug;57:8-16. [4]. Irak degraders and uses thereof. WO/2020/113233. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~114.56 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (2.86 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (2.86 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1456 mL | 5.7278 mL | 11.4557 mL | |
| 5 mM | 0.2291 mL | 1.1456 mL | 2.2911 mL | |
| 10 mM | 0.1146 mL | 0.5728 mL | 1.1456 mL |