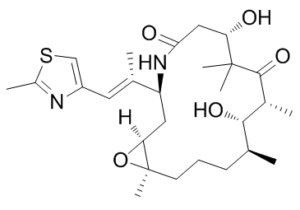
Ixabepilone (formerly known as azaepothilone B and BMS-247550; Trade name: Ixempra) is a potent and orally bioavailable microtubule-stabilizing agent for the treatment of cancer. It attaches itself to tubulin and stimulates microtubule stabilization and tubulin polymerization, which stops cells in their G2-M phase of the cell cycle and causes tumor cells to die. The lactam analogue of epothilone B, BMS-247550 (ixabepilone), demonstrated enhanced metabolic stability, strong tubulin polymerization activity, and continued efficacy against lines resistant to paclitaxel. Ixabepilone was approved by the Food and Drug Administration in 2007 for the treatment of drug-resistant/refractory metastatic or locally advanced breast cancer, based on its demonstrated efficacy in clinical trials.
Physicochemical Properties
| Molecular Formula | C27H42N2O5S |
| Molecular Weight | 506.6978 |
| Exact Mass | 506.281 |
| Elemental Analysis | C, 64.00; H, 8.35; N, 5.53; O, 15.79; S, 6.33 |
| CAS # | 219989-84-1 |
| Related CAS # | 219989-84-1 |
| PubChem CID | 6445540 |
| Appearance | white solid powder |
| Density | 1.1±0.1 g/cm3 |
| Boiling Point | 697.8±55.0 °C at 760 mmHg |
| Flash Point | 375.8±31.5 °C |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.533 |
| LogP | 1.77 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 35 |
| Complexity | 817 |
| Defined Atom Stereocenter Count | 7 |
| SMILES | S1C(C([H])([H])[H])=NC(=C1[H])/C(/[H])=C(\C([H])([H])[H])/[C@]1([H])C([H])([H])[C@@]2([H])[C@@](C([H])([H])[H])(C([H])([H])C([H])([H])C([H])([H])[C@]([H])(C([H])([H])[H])[C@@]([H])([C@@]([H])(C([H])([H])[H])C(C(C([H])([H])[H])(C([H])([H])[H])[C@]([H])(C([H])([H])C(N1[H])=O)O[H])=O)O[H])O2 |
| InChi Key | FABUFPQFXZVHFB-PVYNADRNSA-N |
| InChi Code | InChI=1S/C27H42N2O5S/c1-15-9-8-10-27(7)22(34-27)12-20(16(2)11-19-14-35-18(4)28-19)29-23(31)13-21(30)26(5,6)25(33)17(3)24(15)32/h11,14-15,17,20-22,24,30,32H,8-10,12-13H2,1-7H3,(H,29,31)/b16-11+/t15-,17+,20-,21-,22-,24-,27+/m0/s1 |
| Chemical Name | (1S,3S,7S,10R,11S,12S,16R)-7,11-dihydroxy-8,8,10,12,16-pentamethyl-3-[(E)-1-(2-methyl-1,3-thiazol-4-yl)prop-1-en-2-yl]-17-oxa-4-azabicyclo[14.1.0]heptadecane-5,9-dione |
| Synonyms | Azaepothilone B; BMS 2475501; BMS247550; BMS-247550; BMS 247550 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | microtubule(tubulin stabilising) |
| ln Vitro | BMS-247550 is an extremely effective cytotoxic agent that can eradicate cancer cells even at low nanomolar concentrations. It also maintains its antineoplastic properties against human cancers that are either paclitaxel-resistant or inherently insensitive[1]. |
| ln Vivo | BMS-247550 demonstrates antitumor activity that is superior to paclitaxel in both paclitaxel-resistant and -sensitive tumors. The results of this study indicate that BMS-247550 is more effective than paclitaxel in all five paclitaxel-resistant tumors evaluated (four in humans and one in a mouse): the clinically derived paclitaxel-resistant Pat-7 ovarian carcinoma, the tubulin-mutated A2780Tax ovarian carcinoma that is resistant to paclitaxel, the HCT116/VM46 MDR colon carcinoma, the clinically derived paclitaxel-resistant Pat-21 breast carcinoma, and the murine fibrosarcoma M5076. A2780 human ovarian carcinoma, HCT116, and LS174T human colon carcinoma are the three paclitaxel-sensitive human tumor xenografts against which BMS-247550 exhibits antitumor activity comparable to paclitaxel[1]. |
| Enzyme Assay | Published techniques are used to assess the potency of BMS-247550 and paclitaxel in polymerizing tubulin isolated from calf brain. In short, tubulin is added to polymerization buffer at 37°C in microcuvette wells of a Beckman apparatus along with varying concentrations of paclitaxel or BMS-247550 [0.1 M mes, 1 mM EGTA, 0.5 mM MgCl2 (pH 6.6)]. UV spectrophotometer, model number DU 7400. The final concentration of microtubule protein is set at 1.0 mg/mL, and compound concentrations are typically used at 2.5, 5.0, and 10 μM. The instrument's software program calculates the initial slopes of absorbance (A280 nM), which are measured every 10 seconds. |
| Cell Assay | Trypsinization is used to gather HCT116 cells from cultures after they have been exposed to 7.5 nm of BMS-247550 for 1, 2, 4, 8, 16, and 24 hours. Pelletized cells are fixed at −20°C in 80% ethanol. After being stored at −20°C for the entire night, cells are rehydrated using PBS buffer and then incubated with propidium iodide (5 μg/mL) in 0.1% RNase for 15–30 minutes to stain the DNA. The FACS Calibur device is used for the acquisition of fluorescence-activated cell sorters, and Cellquest and Modfit software are used for the analysis. |
| Animal Protocol |
Human tumor xenografts(BALB/c nu/nu nude mice) various concentrations i.v. or p.o. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Mostly fecal and some renal. Following IV administration of a single dose of radiolabeled drug, approximately 86% of the dose was eliminated within 7 days, 65% in feces and 21% in urine. Unchanged ixabepilone accounted for less than 2 and 6% of the dose in feces and urine, respectively. The drug has a terminal elimination half-life of approximately 52 hours (range: 20-72 hours). No accumulation in plasma is expected when the drug is administered once every 3 weeks. Not known whether ixabepilone is distributed into human milk; however, in lactating rats given radiolabeled ixabepilone, concentrations of radioactivity in milk were comparable to those in plasma and declined in parallel with plasma concentrations of the drug. The mean volume of distribution of 40 mg/sq m ixabepilone at steady-state was in excess of 1000 L. In vitro, the binding of ixabepilone to human serum proteins ranged from 67 to 77%, and the blood-to-plasma concentration ratios in human blood ranged from 0.65 to 0.85 over a concentration range of 50 to 5000 ng/mL. Following administration of a single 40 mg/sq m dose of Ixempra in patients with cancer, the mean Cmax was 252 ng/mL (coefficient of variation, CV 56%) and the mean AUC was 2143 ng*hr/mL (CV 48%).Typically Cmax occurred at the end of the 3 hour infusion. In cancer patients, the pharmacokinetics of ixabepilone were linear at doses of 15 to 57 mg/sq m. Metabolism / Metabolites Ixabepilone is extensively metabolized in the liver, principally by oxidative metabolism via the cytochrome P-450 (CYP) isoenzyme 3A4. The drug is eliminated primarily as metabolized drug, with more than 30 inactive metabolites eliminated in urine and feces. No single metabolite accounted for more than 6% of the administered dose. Biological Half-Life 52 hours The drug has a terminal elimination half-life of approximately 52 hours (range: 20-72 hours). |
| Toxicity/Toxicokinetics |
Hepatotoxicity In preregistration controlled trials, serum aminotransferase elevations and other liver test abnormalities were rarely mentioned. A high proportion of patients treated had mild-to-moderate serum enzyme elevations at the time of starting ixabepilone, probably because of hepatic metastases and the use of other antineoplastic agents. During ixabepilone therapy, worsening of serum enzyme elevations occurred in up to 15% of patients, but ALT elevations above 5 times the upper limit of normal were rare, and there were no reports of severe hepatic adverse events or discontinuations because of enzyme elevations or clinically apparent liver disease. Nevertheless, jaundice and acute liver failure as well as elevations in serum ALT, AST, alkaline phosphatase, and bilirubin are mentioned as occurring in clinical trials in the product label. Since the approval and more widescale use of ixabepilone, there have been no publications or descriptions of the clinical features of hepatotoxicity with jaundice associated with its use. Thus, clinically apparent liver injury probably occurs in a small proportion of patients receiving ixabepilone, but its relationship with the drug is unclear. Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury). Protein Binding 67-77% Interactions At clinically relevant plasma concentrations, ixabepilone does not inhibit CYP isoenzymes 3A4, 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, or 2D6; pharmacokinetic interaction unlikely when ixabepilone is used with substrates of these isoenzymes. Since St. John's wort (Hypericum perforatum) may cause unpredictable decreases in plasma ixabepilone concentrations, such concomitant use should be avoided. Potential pharmacokinetic interaction (decreased and possibly subtherapeutic plasma ixabepilone concentrations) may occur during concomitant use with potent CYP3A4 inducers (e.g., carbamazepine, dexamethasone, phenobarbital, phenytoin, rifabutin, rifampin). If concurrent administration of other drugs is indicated during ixabepilone therapy, drugs with a low enzyme induction potential should be considered. Because the effect of mild or moderate CYP3A4 inhibitors (e.g., erythromycin, fluconazole, verapamil) on exposure to ixabepilone has not been studied, caution should be used during concomitant administration of these drugs and use of alternative therapeutic agents that do not inhibit CYP3A4 should be considered. Patients receiving CYP3A4 inhibitors during ixabepilone therapy should be closely monitored for acute toxicity (e.g., frequent monitoring of peripheral blood counts between cycles of ixabepilone). For more Interactions (Complete) data for Ixabepilone (8 total), please visit the HSDB record page. |
| References |
[1]. John T. Hunt Discovery of Ixabepilone. Mol Cancer Ther February 2009 8; 275 |
| Additional Infomation |
Ixabepilone is a macrocycle that is a lactam analogue of epothilone B. Binds directly to beta-tubulin subunits on microtubules, leading to suppression of microtubule dynamics. It has a role as an antineoplastic agent and a microtubule-destabilising agent. It is a member of 1,3-thiazoles, a beta-hydroxy ketone, a lactam, a macrocycle and an epoxide. Ixabepilone is an epothilone B analog developed by Bristol-Myers Squibb as a cancer drug. It was FDA approved on October 16, 2007, for the treatment of unresponsive aggressive metastatic or locally advanced breast cancer. Ixabepilone is administered through injection, and will be marketed under the trade name Ixempra. Ixabepilone is a semisynthetic analogue of epothilone B. It has a lactone–lactam modification that minimizes susceptibility to esterase degradation. Ixabepilone is a Microtubule Inhibitor. The physiologic effect of ixabepilone is by means of Microtubule Inhibition. Ixabepilone is a semisynthetic epothilone analogue that acts to stabilize microtubules thereby preventing mitosis and causing growth arrest in cancer cells. Ixabepilone is approved for use in refractory cases of advanced breast cancer. Its use is associated with a low rate of serum enzyme elevation, but ixabepilone has not been linked to cases of clinically apparent liver injury with jaundice. Ixabepilone is an orally bioavailable semisynthetic analogue of epothilone B with antineoplastic activity. Ixabepilone binds to tubulin and promotes tubulin polymerization and microtubule stabilization, thereby arresting cells in the G2-M phase of the cell cycle and inducing tumor cell apoptosis. This agent demonstrates antineoplastic activity against taxane-resistant cell lines. Drug Indication Investigated for use/treatment in breast cancer, head and neck cancer, melanoma, lung cancer, lymphoma (non-hodgkin's), prostate cancer, renal cell carcinoma, and cancer/tumors (unspecified). Mechanism of Action Binding of Ixabepilone to beta-tubulins (e.g. beta-III tubulin) stabilizes microtubules. Microtubules are essential to cell division, and epothilones therefore stop cells from properly dividing. Like taxol, Ixabepilone binds to the αβ-tubulin heterodimer subunit. Once bound, the rate of αβ-tubulin dissociation decreases, thus stabilizing the microtubules. Ixabepilone is a microtubule inhibitor belonging to the epothilone class of antineoplastic agents. Epothilones are naturally occurring products of fermentation from the myxobacterium Sorangium cellulosum. Ixabepilone is a semisynthetic derivative of epothilone B, a 16-membered polyketide macrolide, with a chemically modified lactam substitution for the naturally existing lactone. Ixabepilone binds to beta-tubulin subunits on microtubules and stabilizes and suppresses microtubule activity resulting in mitotic arrest and apoptosis. Although ixabepilone appears to share a similar antimicrotubule mechanism of action with taxanes, the drug differs structurally from taxanes and does not appear to be affected by common mechanisms of taxane resistance. Therapeutic Uses Ixabepilone is used in combination with oral capecitabine for the treatment of metastatic or locally advanced breast cancer in patients whose disease is resistant to treatment with an anthracycline and a taxane or in patients whose cancer is taxane-resistant and for whom further anthracycline therapy is contraindicated. Anthracycline resistance is defined as progression during therapy or within 6 months in the adjuvant setting or 3 months in the metastatic setting. Taxane resistance is defined as progression during therapy or within 12 months in the adjuvant setting or 4 months in the metastatic setting. /Included in US product label/ Ixabepilone is used as monotherapy for the treatment of metastatic or locally advanced breast cancer in patients whose tumors are resistant or refractory to anthracyclines, taxanes, and capecitabine. /Included in US product label/ Chemotherapy efficacy in patients with solid tumors is influenced by primary and acquired multidrug resistance (MDR). Epothilones represent a novel class of microtubule inhibitors with lower susceptibility to drug resistance and efficacy in taxane-resistant tumors. While other epothilones are currently under investigation, ixabepilone is the first epothilone B analogue approved by the U.S. Food and Drug Administration. Ixabepilone has been shown to have preclinical activity in chemotherapy-sensitive and chemotherapy-resistant tumor models, and synergistic antitumor activity with other chemotherapeutic and targeted agents. Single-agent ixabepilone has demonstrated clinical activity in multiple solid tumors including advanced breast, lung, prostate, pancreatic, renal cell, and ovarian cancers. Most notably, efficacy has been demonstrated in patients with metastatic breast cancer (MBC) progressing after treatment with anthracyclines and taxanes. A phase III trial in anthracycline- and taxane-resistant MBC showed superior disease control with ixabepilone plus capecitabine versus capecitabine monotherapy, resulting in its approval. Ixabepilone is also active in chemotherapy-naive and taxane-resistant hormone-refractory prostate cancer and platinum-resistant non-small cell lung cancer. Neutropenia and peripheral sensory neuropathy are the most common adverse events associated with treatment. ... Drug Warnings Myelosuppression is one of the major and dose-limiting adverse effects of ixabepilone and is primarily manifested as neutropenia. In clinical studies, grade 4 neutropenia (less than 500 cells/cu mm) occurred in 36% of patients treated with ixabepilone plus capecitabine and 23% of patients treated with ixabepilone monotherapy. Febrile neutropenia and infection with neutropenia were reported in 5 and 6%, respectively, of patients treated with ixabepilone plus capecitabine, and 3 and 5%, respectively, of patients treated with ixabepilone monotherapy. Neutropenia-related death occurred in 1.9% of patients with normal hepatic function or mild hepatic impairment treated with ixabepilone plus capecitabine. The incidence of neutropenia-related death was higher (29%) in patients with serum AST or ALT concentrations exceeding 2.5 times the upper limit of normal or serum bilirubin concentrations exceeding 1.5 times the upper limit of normal. Neutropenia-related death occurred in 0.4% of patients receiving ixabepilone monotherapy. No neutropenia-related deaths were reported in patients with serum AST or ALT concentrations exceeding 2.5 times the upper limit of normal or serum bilirubin concentrations exceeding 1.5 times the upper limit of normal treated with ixabepilone monotherapy. Patients with baseline AST or ALT >2.5 x ULN or bilirubin >1.5 x ULN experienced greater toxicity than patients with baseline AST or ALT = 2.5 x ULN or bilirubin =1.5 x ULN when treated with Ixempra at 40 mg/sq m in combination with capecitabine or as monotherapy in breast cancer studies. In combination with capecitabine, the overall frequency of grade 3/4 adverse reactions, febrile neutropenia, serious adverse reactions, and toxicity related deaths was greater. With monotherapy, grade 4 neutropenia, febrile neutropenia, and serious adverse reactions were more frequent. The safety and pharmacokinetics of Ixempra as monotherapy were evaluated in a dose escalation study in 56 patients with varying degrees of hepatic impairment. Exposure was increased in patients with elevated AST or bilirubin. Ixempra in combination with capecitabine is contraindicated in patients with AST or ALT >2.5 x /Upper Limit of Normal/ (ULN) or bilirubin >1 x ULN due to increased risk of toxicity and neutropenia-related death. Patients who are treated with Ixempra as monotherapy should receive a reduced dose depending on the degree of hepatic impairment. Use in patients with AST or ALT >10 x ULN or bilirubin >3 x ULN is not recommended. Limited data are available for patients with AST or ALT >5 x ULN. Caution should be used when treating these patients. Peripheral neuropathy, mostly sensory in nature but also motor neuropathy, occurs commonly in ixabepilone-treated patients and was reported in over 60% of patients receiving the drug in controlled studies. Although generally mild to moderate in severity, grade 3 or 4 neuropathy was reported in 14 and 23% of patients receiving ixabepilone monotherapy and ixabepilone combined with capecitabine, respectively, in controlled trials. Neuropathy generally develops early during treatment, with approximately 75% of new-onset or worsening neuropathy occurring during the first 3 cycles. Peripheral neuropathy was often characterized as paresthesia or dysesthesia and manifested as a symmetrical, stocking-and-glove distribution with more pronounced effects in the lower extremities. For more Drug Warnings (Complete) data for Ixabepilone (19 total), please visit the HSDB record page. |
Solubility Data
| Solubility (In Vitro) |
DMSO: 83.3~100 mg/mL (164.5~197.4 mM) Ethanol: ~47 mg/mL (~92.8 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (4.10 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (4.10 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (4.10 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 2% DMSO+30% PEG 300+2% Tween 80+ddH2O: 7mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9736 mL | 9.8678 mL | 19.7355 mL | |
| 5 mM | 0.3947 mL | 1.9736 mL | 3.9471 mL | |
| 10 mM | 0.1974 mL | 0.9868 mL | 1.9736 mL |