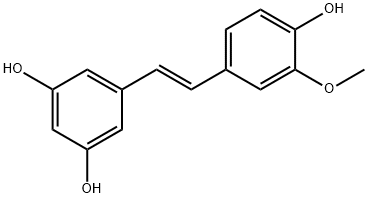
Isorhapontigenin is a novel and potent dietary polyphenol isolated from Gnetum cleistostachyum. Isorhapontigenin shows anti-inflammatory effects. Isorhapontigenin is an inducer of autophagy and blocks invasive bladder cancer formation.
Physicochemical Properties
| Molecular Formula | C15H14O4 |
| Molecular Weight | 258.26926 |
| Exact Mass | 258.089 |
| Elemental Analysis | C, 69.76; H, 5.46; O, 24.78 |
| CAS # | 32507-66-7 |
| PubChem CID | 5318650 |
| Appearance | White to yellow solid |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 471.8±35.0 °C at 760 mmHg |
| Melting Point | 182 - 184ºC |
| Flash Point | 239.1±25.9 °C |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.722 |
| Source | Chinese herb Gnetum cleistostachyum |
| LogP | 2.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 19 |
| Complexity | 295 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | COC1=C(O)C=CC(/C=C/C2=CC(O)=CC(O)=C2)=C1 |
| InChi Key | ANNNBEZJTNCXHY-NSCUHMNNSA-N |
| InChi Code | InChI=1S/C15H14O4/c1-19-15-8-10(4-5-14(15)18)2-3-11-6-12(16)9-13(17)7-11/h2-9,16-18H,1H3/b3-2+ |
| Chemical Name | 5-[(E)-2-(4-hydroxy-3-methoxyphenyl)ethenyl]benzene-1,3-diol |
| Synonyms | Isorhapontigenin; 32507-66-7; Isorhapotogenin; 3'-Methoxyresveratrol; Isorhapotigenin; 5-[(E)-2-(4-hydroxy-3-methoxyphenyl)ethenyl]benzene-1,3-diol; CZ49V3K5HS; 1,3-Benzenediol, 5-[(1E)-2-(4-hydroxy-3-methoxyphenyl)ethenyl]-; 5-[(E)-2-(4-hydroxy-3-methoxyphenyl)ethenyl]benzene-1,3-diol; CZ49V3K5HS; (E)-5-(4-hydroxy-3-methoxystyryl)benzene-1,3-diol; 5-[2-(4-Hydroxy-3-methoxyphenyl)ethenyl]-1,3-benzenediol; 1,3-Benzenediol, 5-[(1E)-2-(4-hydroxy-3-methoxyphenyl)ethenyl]-; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Bioactive natural product;anti‐inflammatory agent |
| ln Vitro |
Isorhapontigenin, an orally bioavailable dietary polyphenol, displayed superior anti‐inflammatory effects compared with resveratrol. Furthermore, it suppressed the PI3K/Akt pathway that is insensitive to corticosteroids. Isorhapontigenin concentration‐dependently inhibited IL‐6 and CXCL8 release, with IC50 values at least twofold lower than those of resveratrol. These were associated with reduced activation of NF‐κB and AP‐1 and, notably, the PI3K/Akt/FoxO3A pathway, that was relatively insensitive to dexamethasone.[2] Isorhapontigenin concentration-dependently inhibited IL-6 and CXCL8 release, with IC50 values at least twofold lower than those of resveratrol. These were associated with reduced activation of NF-κB and AP-1 and, notably, the PI3K/Akt/FoxO3A pathway, that was relatively insensitive to dexamethasone.[1] Isorhapontigenin (ISO) is a new derivative of stilbene isolated from the Chinese herb Gnetum cleistostachyum. Our recent studies have revealed that ISO treatment at doses ranging from 20 to 80 μM triggers apoptosis in multiple human cancer cell lines. In the present study, we evaluated the potential effect of ISO on autophagy induction. We found that ISO treatment at sublethal doses induced autophagy effectively in human bladder cancer cells, which contributed to the inhibition of anchorage-independent growth of cancer cells. In addition, our studies revealed that ISO-mediated autophagy induction occurred in a SESN2 (sestrin 2)-dependent and BECN1 (Beclin 1, autophagy related)-independent manner. Furthermore, we identified that ISO treatment induced SESN2 expression via a MAPK8/JNK1 (mitogen-activated protein kinase 8)/JUN-dependent mechanism, in which ISO triggered MAPK8-dependent JUN activation and facilitated the binding of JUN to a consensus AP-1 binding site in the SESN2 promoter region, thereby led to a significant transcriptional induction of SESN2. Importantly, we found that SESN2 expression was dramatically downregulated or even lost in human bladder cancer tissues as compared to their paired adjacent normal tissues. Collectively, our results demonstrate that ISO treatment induces autophagy and inhibits bladder cancer growth through MAPK8-JUN-dependent transcriptional induction of SESN2, which provides a novel mechanistic insight into understanding the inhibitory effect of ISO on bladder cancers and suggests that ISO might act as a promising preventive and/or therapeutic drug against human bladder cancer[2]. |
| ln Vivo |
In vivo, isorhapontigenin was rapidly absorbed with abundant plasma levels after oral dosing. Its oral bioavailability was approximately 50% higher than resveratrol.[2]
ISO treatment inhibited BBN-induced mouse invasive BC formation in vivo [3] Isorhapontigenin (ISO) has been shown to inhibit growth and induce apoptosis in human BC cells in our recent studies. N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) is a well-characterized bladder carcinogen for its induction of 100% invasive BC in mouse model. To explore whether ISO exhibit BC invasion, we first employed BBN-induced invasive BC mouse model and examined the effects of ISO on BBN-induced mouse invasive BC formation. As shown in Table 1 and Figure 1A, none of the vehicle-treated control mice developed BC, whereas BBN induced 100% (12/12) high-grade muscle-invasive BCs formation. Interestingly, only 16.7% (2/12) of the BBN-treated mice developed high-grade muscle-invasive BC while ISO was administrated, with 7 cases of papillomas and 3 cases of low-grade non-muscle-invasive BCs, demonstrating a novel biological activity of ISO as an efficient drug that targets at stage of invasive BC development in vivo (p<0.05). |
| Enzyme Assay |
Luciferase assay [3] As described in our previous studies, dual-luciferase reporter assay was performed by using the luciferase assay system. Briefly, Human FasL promoter, IGFBG-1 (3×IRS) promoter, FOXO1 promoter, and MMP-2 promoter luciferase reporters were transfected into the indicated human BC cells, respectively. After Isorhapontigenin/ISO treatment, cells were extracted with passive lysis buffer [25 mM Tris-phosphate (pH 7.8), 2 mM EDTA, 1% Triton X-100, and 10% glycerol]. The luciferase activity was measured with a microplate luminometer LB 96V. The Renilla luciferase signal was normalized to the internal firefly luciferase transfection control. |
| Cell Assay |
Primary human airway epithelial cells derived from healthy and COPD subjects, and A549 epithelial cells were incubated with isorhapontigenin or resveratrol and stimulated with IL‐1β in the presence or absence of cigarette smoke extract. Effects of isorhapontigenin and resveratrol on the release of IL‐6 and chemokine (C‐X‐C motif) ligand 8 (CXCL8), and the activation of NF‐κB, activator protein‐1 (AP‐1), MAPKs and PI3K/Akt/FoxO3A pathways were determined and compared with those of dexamethasone. [2]
Anchorage-independent growth assay [2] The soft-agar assay was performed as described previously.6,38 Briefly, 2.5 ml of 0.5% agar in basal modified Eagle's medium supplemented with 10% FBS with or without IsorhapontigeninISO was layered onto each well of 6-well tissue culture plates. 1 × 104 UMUC3 cells or their transfectants were mixed with 1 ml of 0.5% agar in basal modified Eagle's medium supplemented with 10% FBS with or without ISO, and then layered on top of the 0.5% agar layer. The plates were incubated at 37°C in 5% CO2 for 2 weeks. The colonies with more than 32 cells were scored and the results were presented as colonies/104 cells. ATP cell viability assay [3] Cell viability was measured utilizing CellTiter-Glo Luminescent Cell Viability Assay Kit according to the manufacturer’s instructions as described in our previous studies. Briefly, Cells were plated in 96-well plates at a density of 10000 cells per well and allowed to adhere overnight. The cell culture medium was then replaced with 0.1% FBS DMEM and cultured for 12 hours. After Isorhapontigenin/ISO treatment for the indicated time and doses, 50 μl of CellTiter-Glo assay reagent was added to each well. The contents were mixed on an orbital shaker for 2 minutes to induce cell lysis, and then incubated at room temperature for 10 minutes to stabilize the luminescent signal. Results were read on a microplate luminometer LB 96V. Cell viability (%) was defined as the relative absorbance of treated samples versus that of the untreated control. All experiments were performed with six wells for each experiment and repeated at least three times. In vitro cellular migration and invasion assays [3] In vitro migration and invasion assays were conducted by using transwell chamber (for migration assay) or transwell pre-coated matrigel chamber (for invasion assay) according to the manufacturer's protocol as described previously. Briefly, 700 μl of medium containing 10% FBS (for UMUC3 and T24T cells) or 40% FBS (for RT4 cells) was added to the lower chambers, while homogeneous single cell suspensions (5×104 cells/well) in 0.1% FBS medium with or without Isorhapontigenin/ISO as indicated were added to the upper chambers. The transwell plates were incubated in 5% CO2 incubator at 37°C for 24 hours, and thereafter were washed by PBS, fixed with 4% formaldehyde, and stained with Giemsa stain. The non-migration or non-invading cells were scrapped off on the top of chamber. The migration and invasion rates were quantified by counting the migration and invaded cells at least three random fields under a light microscope. Western blotting [3] Western blot assay was assessed as previously described. Briefly, cells were plated in 6-well plates and cultured in normal FBS medium until 70–80% confluence. The cells were then cultured in 0.1% FBS medium for 12 hours, followed by treatment with different doses of Isorhapontigenin/ISO for the indicated time. The cells were washed once with ice-cold phosphate-buffered saline and cell lysates were prepared with a lysis buffer (10 mM Tris-HCl (pH 7.4), 1% SDS, and 1 mM Na3VO4). An equal amount (80 μg) of total protein from each cell lysate was subjected to Western blot with the indicated antibody as described in previous studies. Immunoreactive bands were detected by using the alkaline phosphatase-linked secondary antibody and ECF Western blotting system. The images were acquired by using Typhoon FLA 7000 imager. |
| Animal Protocol |
The pharmacokinetic profiles of Isorhapontigenin, after i.v. or oral administration, were assessed in Sprague–Dawley rats.[2] Pharmacokinetic study [2] The Isorhapontigenin was prepared for i.v. administration by dissolving the powder in 0.3 M 2‐hydroxypropyl β‐cyclodextrin 1 h before dosing while the oral suspension was prepared by suspending the powder in 0.3% (w.v‐1) carboxymethylcellulose just before dosing. L‐ascorbic acid [final concentration = 0.01% (w.v‐1)] was added to both formulations to ensure stability of the compound.[2] Five rats received a single bolus i.v. injection of Isorhapontigenin at the dose of 30 μmol·kg−1 (7.74 mg·kg−1) while the other five rats received a single dose of isorhapontigenin 600 μmol·kg−1 (154.8 mg·kg−1), p.o. Serial blood samples were collected before dosing and at 5, 15, 30, 60, 90, 120, 180, 240, 300, 420 and 600 min after i.v. administration and at 15, 30, 45, 60, 90, 120, 180, 240, 300, 420, 540 and 720 min after p.o. administration. The blood samples were collected in tubes containing L‐ascorbic acid (final concentration = 0.8 mg·mL−1) to ensure stability. The samples were centrifuged at 5000 g for 5 min, and the plasma layer was retrieved and stored at −80°C until analysed. Following the study period, the animals were killed by exposure to CO2.[2] The C57BL/6J male mice at age of 5~6 weeks were randomly divided into three groups, 12 mice in each group, including vehicle-treated control group, N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN)-treated group, and BBN combined with Isorhapontigenin/ISO-treated group. Mice in BBN-treated group were received BBN (0.05%) in drinking water for 20 weeks, while mice in BBN combined with ISO-treated group were received BBN and ISO (150 mg/kg/day) in drinking water for 20 weeks. ISO was given to the mice in drinking water on the day of initial exposure to BBN, and continued throughout the tumor induction period. Mice bladder tissues were excised and fixed overnight in 4% paraformaldehyde at 4°C. Fixed tissues were processed for paraffin embedding, and the serial 5 μm thick sections were then stained by Hematoxylin and eosin staining (HE). [3] |
| ADME/Pharmacokinetics |
Pharmacokinetics of isorhapontigenin in rats [1] Upon bolus i.v. administration of isorhapontigenin at 30 μmol·kg−1 (7.74 mg·kg−1) in Sprague–Dawley rats, plasma isorhapontigenin levels first declined rapidly before a more prolonged terminal elimination phase where secondary peaks were observed from 90 min (Figure 7A). Appearance of secondary peaks suggests the involvement of enterohepatic recirculation, which has also been reported for resveratrol (Marier et al., 2002; Chen et al., 2016). Isorhapontigenin was eliminated rapidly, and its plasma level dropped below the lower limit of quantification (1 ng·mL−1 or 3.87 nM) of the LC‐MS/MS method in one sample collected at 90 min and two samples collected at 300 min post‐injection. However, isorhapontigenin became measurable again in the next sample collected from the same rat, probably due to the enterohepatic circulation. Such erratic pharmacokinetic behaviour makes the accurate calculation of plasma exposure (AUC0→last) and mean transition time (MTT0→last) difficult. To facilitate the estimation of AUC0→t and MTT, the plasma level of isorhapontigenin in these three samples was adjusted to the lower limit of quantification (1 ng·mL−1 or 3.87 nM). Of note, such approximation would overestimate the i.v. AUC0→t and MTT0→t to a very low extent, and it was also applied in a recent pharmacokinetic study of resveratrol (Chen et al., 2016). Isorhapontigenin was found to display a moderate V, MTT0→last and rather rapid clearance (Cl) (Table 3). When isorhapontigenin was administered through oral gavage as a suspension at 600 μmol·kg−1 (154.8 mg·kg−1), it was absorbed rapidly (Figure 7B), achieving maximal plasma concentration (Cmax) within the first hour after dosing. Isorhapontigenin was measurable in all post‐dosing plasma samples. The AUC0→last of isorhapontigenin was fairly abundant, and the absolute oral bioavailability (F) was relatively high (Table 3). Similar to i.v. dosing, secondary peaks were also observed from 180 min post‐dosing. Such phenomena have also been observed after oral administration of resveratrol and oxyresveratrol (Chen et al., 2016). |
| References |
[1]. Isorhapontigenin, a bioavailable dietary polyphenol, suppresses airway epithelial cell inflammation through a corticosteroid-independent mechanism. Br J Pharmacol. 2017 Jul;174(13):2043-2059. [2]. SESN2/sestrin 2 induction-mediated autophagy and inhibitory effect of isorhapontigenin (ISO) on human bladder cancers. Autophagy. 2016 Aug 2;12(8):1229-39. [3]. Isorhapontigenin (ISO) Inhibits Invasive Bladder Cancer Formation In Vivo and Human Bladder Cancer Invasion In Vitro by Targeting STAT1/FOXO1 Axis. Cancer Prev Res (Phila). 2016 Jul;9(7):567-80. |
| Additional Infomation |
Isorhapontigenin is a stilbenoid. Isorhapontigenin has been reported in Gnetum montanum, Gnetum pendulum, and other organisms with data available. Although our most recent studies have identified Isorhapontigenin (ISO), a novel derivative of stilbene that isolated from a Chinese herb Gnetum cleistostachyum, for its inhibition of human bladder cancer growth, nothing is known whether ISO possesses an inhibitory effect on bladder cancer invasion. Thus, we addressed this important question in current study and discovered that ISO treatment could inhibit mouse-invasive bladder cancer development following bladder carcinogen N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN) exposure in vivo We also found that ISO suppressed human bladder cancer cell invasion accompanied by upregulation of the forkhead box class O 1 (FOXO1) mRNA transcription in vitro Accordingly, FOXO1 was profoundly downregulated in human bladder cancer tissues and was negatively correlated with bladder cancer invasion. Forced expression of FOXO1 specifically suppressed high-grade human bladder cancer cell invasion, whereas knockdown of FOXO1 promoted noninvasive bladder cancer cells becoming invasive bladder cancer cells. Moreover, knockout of FOXO1 significantly increased bladder cancer cell invasion and abolished the ISO inhibition of invasion in human bladder cancer cells. Further studies showed that the inhibition of Signal transducer and activator of transcription 1 (STAT1) phosphorylation at Tyr701 was crucial for ISO upregulation of FOXO1 transcription. Furthermore, this study revealed that metalloproteinase-2 (MMP-2) was a FOXO1 downstream effector, which was also supported by data obtained from mouse model of ISO inhibition BBN-induced mouse-invasive bladder cancer formation. These findings not only provide a novel insight into the understanding of mechanism of bladder cancer's propensity to invasion, but also identify a new role and mechanisms underlying the natural compound ISO that specifically suppresses such bladder cancer invasion through targeting the STAT1-FOXO1-MMP-2 axis.[3] Background and purpose: Chronic obstructive pulmonary disease (COPD) is a corticosteroid-resistant airway inflammatory condition. Resveratrol exhibits anti-inflammatory activities in COPD but has weak potency and poor pharmacokinetics. This study aimed to evaluate the potential of isorhapontigenin, another dietary polyphenol, as a novel anti-inflammatory agent for COPD by examining its effects in vitro and pharmacokinetics in vivo. Experimental approach: Primary human airway epithelial cells derived from healthy and COPD subjects, and A549 epithelial cells were incubated with isorhapontigenin or resveratrol and stimulated with IL-1β in the presence or absence of cigarette smoke extract. Effects of isorhapontigenin and resveratrol on the release of IL-6 and chemokine (C-X-C motif) ligand 8 (CXCL8), and the activation of NF-κB, activator protein-1 (AP-1), MAPKs and PI3K/Akt/FoxO3A pathways were determined and compared with those of dexamethasone. The pharmacokinetic profiles of isorhapontigenin, after i.v. or oral administration, were assessed in Sprague-Dawley rats. Key results: Isorhapontigenin concentration-dependently inhibited IL-6 and CXCL8 release, with IC50 values at least twofold lower than those of resveratrol. These were associated with reduced activation of NF-κB and AP-1 and, notably, the PI3K/Akt/FoxO3A pathway, that was relatively insensitive to dexamethasone. In vivo, isorhapontigenin was rapidly absorbed with abundant plasma levels after oral dosing. Its oral bioavailability was approximately 50% higher than resveratrol. Conclusions and implications: Isorhapontigenin, an orally bioavailable dietary polyphenol, displayed superior anti-inflammatory effects compared with resveratrol. Furthermore, it suppressed the PI3K/Akt pathway that is insensitive to corticosteroids. These favourable efficacy and pharmacokinetic properties support its further development as a novel anti-inflammatory agent for COPD.[1] |
Solubility Data
| Solubility (In Vitro) | DMSO : ~50 mg/mL (~193.60 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (9.68 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (9.68 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (9.68 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.8719 mL | 19.3596 mL | 38.7192 mL | |
| 5 mM | 0.7744 mL | 3.8719 mL | 7.7438 mL | |
| 10 mM | 0.3872 mL | 1.9360 mL | 3.8719 mL |