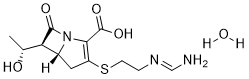
Imipenem belongs to the carbapenem class of antibiotics that is isolated from the soil organism Streptomyces cattleya, and is an intravenous β-lactam antibiotic discovered by Merck scientists Burton Christensen, William Leanza, and Kenneth Wildonger in 1980. It was the first member of the carbapenem class of antibiotics. Carbapenems are highly resistant to the β-lactamase enzymes produced by many multiple drug-resistant Gram-negative bacteria, and thus play a key role in the treatment of infections not readily treated with other antibiotics.
Physicochemical Properties
| Molecular Formula | C12H19N3O5S |
| Molecular Weight | 317.3614 |
| Exact Mass | 317.104 |
| Elemental Analysis | C, 45.42; H, 6.03; N, 13.24; O, 25.21; S, 10.10 |
| CAS # | 74431-23-5 |
| Related CAS # | Imipenem;64221-86-9 |
| PubChem CID | 5282372 |
| Appearance | Off-white to yellow solid powder |
| Density | 1.02 g/mL at 25 °C |
| Boiling Point | 34.6°C |
| Melting Point | -116.3°C |
| Flash Point | <−30 °F |
| LogP | 0.188 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 21 |
| Complexity | 491 |
| Defined Atom Stereocenter Count | 3 |
| SMILES | S(C([H])([H])C([H])([H])/N=C(\[H])/N([H])[H])C1=C(C(=O)O[H])N2C([C@]([H])([C@@]([H])(C([H])([H])[H])O[H])[C@@]2([H])C1([H])[H])=O.O([H])[H] |
| InChi Key | GSOSVVULSKVSLQ-JJVRHELESA-N |
| InChi Code | InChI=1S/C12H17N3O4S.H2O/c1-6(16)9-7-4-8(20-3-2-14-5-13)10(12(18)19)15(7)11(9)17/h5-7,9,16H,2-4H2,1H3,(H2,13,14)(H,18,19)1H2/t6-,7-,9-/m1./s1 |
| Chemical Name | (5R,6S)-3-((2-((E)-(aminomethylene)amino)ethyl)thio)-6-((R)-1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid hydrate |
| Synonyms | Primaxin, MK-0787; MK 0787; MK0787; Imipenem monohydrate; 74431-23-5; Imipenem hydrate; Tienam; (5R,6S)-3-((2-Formimidamidoethyl)thio)-6-((R)-1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid hydrate; Primaxin; Imipenem, Monohydrate; 74431-23-5 (hydrate); MK-787; MK787; MK 787; N-Formimidoylthienamycin; Tienamycin; Imipemide; Imipenem hydrate; Recarbrio; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product is not stable in solution, please use freshly prepared working solution for optimal results. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam antibiotic; bacerial cell wall synthesis |
| ln Vitro | The in vitro activity of N-formimidoyl thienamycin (MK0787), a stable congener of thienamycin, was determined against 200 species of aerobic and 84 species of anaerobic bacteria. The compound was highly active against resistant gram-negative bacilli, penicillin-resistant Staphylococcus aureus, enterococci, and anaerobic bacteria. The new derivative of thienamycin was more active than the parent compound, probably reflecting the stability of the analog[2]. |
| ln Vivo |
Time-dependent death is the effect of imipenem (MK0787) (4 mg/kg, 8 mg/kg, 16 mg/kg, 32 mg/kg, 64 mg/kg, IP, single dose) [3]. Ipenem's pharmacokinetic properties (4 mg/kg, 8 mg/kg, 16 mg/kg, 32 mg/kg, 64 mg/kg, IP,single) in a neutropenic mouse biofilm lung infection model[1]. 50 Drugs and dosage (mg/kg) Cmax (mg/L) Tmax (min) AUCtot (mg·min/L) Vz/F (ml/kg) Vss/F (ml/kg) CL/F (ml/min ) /kg) t1/2(min) MRT(min) Imipenem8 15 (7.1) 21 (11) 1,470 (777) 648 (330) 721 (343) 6.7 (3) 67 (11) 108 (12) 16 34 (6) 28 (18) 2,857 (559) 507 (140) 543 (121) 5.8 (1) 60 (9.1) 94 (10) 32 54 (11) 18 (6.1) 4,895 (635) 516 (75 ) 566 (83) 6.6 (0.8) 54 (6.5) 86 (11) 64 69 (37) 15 (9.5) 6,037 (2,976) 547 (274) 617 (308) 7.4 (3.6) 43 (22) 70 (35 ).
Many Pseudomonas aeruginosa isolates from the airways of patients with cystic fibrosis (CF) are sensitive to antibiotics in susceptibility testing, but eradication of the infection is difficult. The main reason is the biofilm formation in the airways of patients with CF. The pharmacokinetics (PKs) and pharmacodynamics (PDs) of antimicrobials can reliably be used to predict whether antimicrobial regimens will achieve the maximum bactericidal effect against infections. Unfortunately, however, most PK/PD studies of antimicrobials have been done on planktonic cells and very few PK/PD studies have been done on biofilms, partly due to the lack of suitable models in vivo. In the present study, a biofilm lung infection model was developed to provide an objective and quantitative evaluation of the PK/PD profile of antimicrobials. Killing curves were set up to detect the antimicrobial kinetics on planktonic and biofilm P. aeruginosa cells in vivo. Colistin showed concentration-dependent killing, while imipenem showed time-dependent killing on both planktonic and biofilm P. aeruginosa cells in vivo. The parameter best correlated to the elimination of bacteria in lung by colistin was the area under the curve (AUC) versus MIC (AUC/MIC) for planktonic cells or the AUC versus minimal biofilm inhibitory concentration (MBIC; AUC/MBIC) for biofilm cells. The best-correlated parameter for imipenem was the time that the drug concentration was above the MIC for planktonic cells (T(MIC)) or time that the drug concentration was above the MBIC (T(MBIC)) for biofilm cells. However, the AUC/MIC of imipenem showed a better correlation with the efficacy of imipenem for biofilm infections (R(2) = 0.89) than planktonic cell infections (R(2) = 0.38). The postantibiotic effect (PAE) of colistin and imipenem was shorter in biofilm infections than planktonic cell infections in this model[3]. |
| Enzyme Assay | Wild-type P. aeruginosa PAO1 was used in this study. Colistin and imipenem were pharmaceutical grade. The MIC was detected by Etest and by a microtiter method; the minimal bactericidal concentration (MBC) for planktonic cells was detected by the microtiter method. The MICs and MBCs of colistin for PAO1 were 2 to 4 mg/liter and 8 mg/liter, respectively; the MICs and MBCs of imipenem were 1 mg/liter and 4 mg/liter, respectively. The minimal biofilm inhibitory concentration (MBIC) and minimal biofilm eradication concentration (MBEC) of colistin and imipenem were determined by use of a modified Calgary biofilm device as previously reported. In short, the biofilms are formed on pegs, treated by antibiotics, and detached by sonication for the assessment of bacterial killing. The MBIC and MBEC of colistin were 8 mg/liter and 64 mg/liter, respectively; the MBIC and MBEC of imipenem were 8 mg/liter and 128 mg/liter, respectively[3]. |
| Animal Protocol |
Animal/Disease Models: Neutropenic biofilm lung infection mouse model [3] Doses: 4 mg/kg, 8 mg/kg, 16 mg/kg, 32 mg/kg, 64 mg/kg Doses: 4 mg/kg, 8 mg/kg, 16 mg/kg kg, 32 mg/kg, 64 mg/kg, IP, single Doses: Demonstrates time-dependent killing of mice infected with biofilm bacterial lungs effect. PKs of colistin and imipenem.[3] While the infected animals were under anesthesia, they were treated intraperitoneally 2 h after infection with 0.2 ml of different doses of colistin (16 mg/kg, 64 mg/kg, 256 mg/kg; 6 mice/regimen; total, 18 mice) or imipenem (4 mg/kg, 8 mg/kg, 16 mg/kg, 32 mg/kg, 64 mg/kg; 6 mice/regimen; total, 30 mice) as a single administration. The control groups received equal volumes of 0.15 M NaCl intraperitoneally. An approximately 0.08-ml blood sample was collected from the tail at 5 min, 15 min, 30 min, 60 min, 120 min, 180 min, and 240 min after antibiotic administration. At the end of the experiment, the mice were euthanized with pentobarbital/lidocaine. Blood samples were centrifuged at 3,000 rpm, and serum was collected for measurement of antibiotic concentration by a biologic method (agar diffusion), as reported previously, employing Streptococcus sp. strain EB68 (imipenem) or Bordetella bronchiseptica ATCC 4617 (colistin). The detection limits were 1 μg/ml (colistin) and 0.2 μg/ml (imipenem). Data about the variability of the assay are presented in the supplementary material. Time-concentration curves of colistin and imipenem were established. Time-kill study of colistin and imipenem in planktonic and biofilm bacteria and PAE.[3] To establish killing curves of colistin and imipenem, anesthetized neutropenic mice infected with planktonic bacteria (4 mice/point; total, 176 mice) or biofilm bacteria (4 mice/point; total, 176 mice) were treated at 2 h after infection with a single intraperitoneal dose of colistin or imipenem (4× MIC, 16× MIC, and 64× MIC; 4 to 256 mg/kg). Control mice received the same volume of saline. The mice were euthanized, and lungs were collected aseptically at −2, 0, 2, 4, 8, 12, and 24 h after bacterial challenge and homogenized in 5 ml of sterilized saline. Humane endpoints were applied during the period. The numbers of CFU were counted for plotting of the killing curves. The duration of the postantibiotic effect (PAE) was calculated by the formula T − C, where T is the time required for the mean count of CFU in the lung of treated mice to increase by 1 log10 unit above its value at the time that the antibiotic concentration in serum fell below the MIC or MBIC, and C is the time required for the mean count of CFU in the lungs of control mice to increase by 1 log unit above the viable count at time zero. PK/PD indices of colistin and imipenem in planktonic and biofilm bacteria in vivo.[3] To establish PK/PD indices of colistin and imipenem, anesthetized neutropenic mice infected with planktonic bacteria (total, 60 mice) or biofilm bacteria (total, 60 mice) were treated from the time point of 2 h after infection with multiple intraperitoneal doses of colistin (range, 16 to 256 mg/kg, representing 2× MBIC to 32× MBIC/4× MBEC) or imipenem (range, 8 to 64 mg/kg, representing 1× MBIC to 8× MBIC). Due to the toxicity of imipenem, it was not possible to administer higher dosages. The multiple dosages were administered at time intervals ranging from 2 h to 16 h after infection for periods of 12 h (colistin) and 24 h (imipenem). The mice were euthanized, and lungs were collected at the end of the experiment and homogenized in 5 ml of sterilized saline. The numbers of CFU were counted for each lung and expressed as the log10 number of CFU per lung. The counts of viable bacteria for each regimen were plotted with the PK parameters. |
| ADME/Pharmacokinetics |
Absorption Imipenem is not effectively absorbed from the gastrointestinal tract and therefore must be administered parenterally. The bioavailability of the IM injection is 89%. Route of Elimination Approximately 70% of imipenem is excreted in the urine as the parent drug. Volume of Distribution The reported volume of distribution for imipenem ranges from 0.23-0.31 L/kg. Clearance The total clearance of imipenem is 0.2 L/h/kg. When used alone, the renal clearance is 0.05 L/h/kg. In combination with cilastatin the renal clearance of imipenem is 0.15 L/h/kg, likely due to the increased concentration of the parent drug. Metabolism / Metabolites Imipenem is metabolized by renal dehydropeptidase. Renal. Half Life: 1 hour Biological Half-Life When given via IV injection imipenem has a half-life of 1 h. The apparent half-life of the IM injection ranges from 1.3-5.1 h, likely due to slower absorption form the injection site. View More
PKs of colistin and imipenem.[3] PK/PD index determination.[3] The Cmax/MIC, AUC/MIC, and TMIC for colistin and imipenem are presented in Fig. 3 and 4, respectively. The PK/PD indices that best correlated with the in vivo efficacy were AUC/MIC for colistin (R2 = 0.77 in planktonic cell infection and 0.85 in biofilm infection) and TMIC for imipenem (R2 = 0.94 for planktonic cell infection and 0.98 for biofilm infection). The planktonic and biofilm bacterial loads in the lungs of mice were 2.0 × 104 CFU/lung in the beginning and reached 109 CFU/lung after 24 h in the untreated control mice. The AUC/MIC of imipenem showed a good correlation with the efficacy of imipenem treatment of biofilm infections (R2 = 0.89) but a poor correlation for planktonic cell infections (R2 = 0.38). The AUC/MBIC and TMBIC of imipenem and colistin are presented in figures in the supplemental material, which show the time-dependent killing of imipenem (R2 = 0.98) and the AUC-dependent killing of colistin (R2 = 0.85). Relationship between AUC/MIC and TMIC with antibacterial effect.[3] The influences of PK and PD parameters on the antimicrobial effect are presented in Tables 3 and 4. Dose-response data were analyzed to examine the impact of the PK/PD parameters by relating the number of bacteria in lung to AUC/MIC (colistin) and TMIC (imipenem). To determine the PK/PD relationships, the correlations (Hill's equation) in both planktonic and biofilm cells were calculated, and the effect was defined as the decrease in the number of CFU in the lung after the first treatment compared to the number of CFU in untreated control mice (at the 2-h time point). Emax values were estimated to be −4.4 (planktonic cells) versus −2.2 (biofilms) log CFU for colistin and −6.6 (planktonic cells) versus −6.6 (biofilms) log CFU for imipenem. The EC50s (AUC/MIC) were 3,840 (planktonic cells) versus >18,420 (biofilms) for colistin and 3.4 (planktonic cells) versus 4.6 (biofilms) for imipenem (TMIC). The depression of the numbers of CFU in the lung with the PK/PD model parameters of AUC/MIC (colistin) and TMIC (imipenem) is presented in Table 4. The 2-log10-kill effect of colistin required AUC/MIC ratios of 61,980 for biofilm cells and 25,980 for planktonic cells and at least 18 h imipenem treatment for biofilm cells and 10 h for planktonic cells (TMIC). PK-PD simulation in the biofilm infection model of neutropenic mouse.[3] The PK-PD simulation with planktonic and biofilm cells is presented in Table 5. The colistin doses were 16 to 256 mg/kg, and the imipenem doses were 8 to 64 mg/kg. The Cmax/MIC values were 5.4 to 66 for colistin and 15 to 69 for imipenem, but the Cmax/MBEC values were 0.3 to 4.1 for colistin and 0.12 to 0.54 for imipenem. The AUC/MIC values of colistin were 280 to 6,132, while the AUC/MBEC values were 18 to 383. The time that the drug concentration was above the MBEC (TMBEC) was 0 when imipenem was administered at doses of 16 mg/kg to 64 mg/kg. |
| Toxicity/Toxicokinetics |
Toxicity Summary
Imipenem acts as an antimicrobial through the inhibition of cell wall synthesis of various gram-positive and gram-negative bacteria. This inhibition of cell wall synthesis in gram-negative bateria is attained by binding to pencillin binding proteins (PBPs). In E. coli and selected strains of P. aeruginosa, imipenem has shown to have the highest affinity to PBP-2, PBP-1a, and PBP-1b. This preferential binding to PBP-2 and PBP-1b results in the direct conversion of the individual cell to a spheroblast, which leads to rapid cell lysis and death without filament formation. Carcinogen Classification Carcinogen Classification No indication of carcinogenicity to humans (not listed by IARC). Exposure Routes Imipenem is not effectively absorbed from the gastrointestinal tract and therefore must be administered parenterally. Protein Binding Imipenem is 20% bound to plasma proteins. |
| References |
[1]. Johann Motsch, et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin Infect Dis. 2020 Apr 15;70(9):1799-1808. [2]. F P Tally, et al. In vitro activity of N-formimidoyl thienamycin (MK0787). Antimicrob Agents Chemother. 1980 Oct;18(4):642-4. [3]. Wang Hengzhuang, et al. In vivo pharmacokinetics/pharmacodynamics of colistin and imipenem in Pseudomonas aeruginosa biofilm infection. Antimicrob Agents Chemother. 2012 May;56(5):2683-90. |
| Additional Infomation |
Imipenem hydrate is a member of carbapenems. It contains an imipenem. Imipenem is a broad-spectrum, semi-synthetic beta-lactam carbapenem derived from thienamycin, produced by Streptomyces cattleya. Imipenem binds to and inactivates penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. PBPs are enzymes that are involved in the last stages of assembling the bacterial cell wall and in reshaping the cell wall during growth and division. This inactivation results in the weakening of the bacterial cell wall and eventually causes cell lysis. Imipenem has the greatest affinity for PBP 1A, 1B, and 2, and its lethal effect is related to binding to PBP 2 and 1B. This antibiotic is active against a wide range of gram-positive and gram-negative organisms and is stable in the presence of beta-lactamases. (NCI05) Semisynthetic thienamycin that has a wide spectrum of antibacterial activity against gram-negative and gram-positive aerobic and anaerobic bacteria, including many multiresistant strains. It is stable to beta-lactamases. Clinical studies have demonstrated high efficacy in the treatment of infections of various body systems. Its effectiveness is enhanced when it is administered in combination with CILASTATIN, a renal dipeptidase inhibitor. |
Solubility Data
| Solubility (In Vitro) |
H2O : ~7.14 mg/mL (~22.50 mM ) DMSO : < 1 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: 10 mg/mL (31.51 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication (<60°C). (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1510 mL | 15.7550 mL | 31.5100 mL | |
| 5 mM | 0.6302 mL | 3.1510 mL | 6.3020 mL | |
| 10 mM | 0.3151 mL | 1.5755 mL | 3.1510 mL |