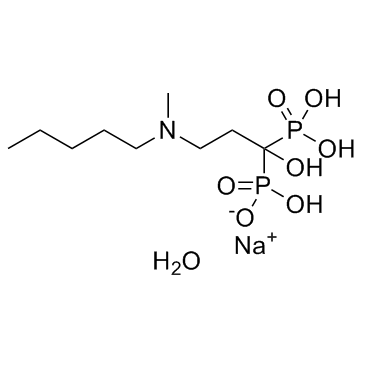
Ibandronate sodium monohydrate (BM210955; RPR102289A) is a bisphosphonate medication used to treat osteoporosis. It works by boosting bone mineral density, stopping resorption, and reducing osteoclast activity.
Physicochemical Properties
| Molecular Formula | C9H24NNAO8P2 |
| Molecular Weight | 359.22 |
| Exact Mass | 359.087 |
| Elemental Analysis | C, 30.09; H, 6.73; N, 3.90; Na, 6.40; O, 35.63; P, 17.24 |
| CAS # | 138926-19-9 |
| Related CAS # | Ibandronic acid;114084-78-5;Ibandronate Sodium;138844-81-2 |
| PubChem CID | 23663991 |
| Appearance | Solid powder |
| Boiling Point | 587.8ºC at 760 mmHg |
| Melting Point | 84ºC (dec) |
| Flash Point | 309.3ºC |
| Vapour Pressure | 2.88E-16mmHg at 25°C |
| LogP | 0.873 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 9 |
| Heavy Atom Count | 21 |
| Complexity | 377 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | OC(P(O)(O)=O)(P(O)([O-])=O)CCN(C)CCCCC.[Na+].O |
| InChi Key | VBDRTGFACFYFCT-UHFFFAOYSA-M |
| InChi Code | InChI=1S/C9H23NO7P2.Na.H2O/c1-3-4-5-7-10(2)8-6-9(11,18(12,13)14)19(15,16)17;;/h11H,3-8H2,1-2H3,(H2,12,13,14)(H2,15,16,17);;1H2/q;+1;/p-1 |
| Chemical Name | sodium;hydroxy-[1-hydroxy-3-[methyl(pentyl)amino]-1-phosphonopropyl]phosphinate;hydrate |
| Synonyms | Ibandronate sodium monohydrate; Boniva; Ibandronate sodium; Sodium trihydrogen (1-hydroxy-3-(methylpentylamino)propylidene)diphosphonate, monohydrate |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Ibandronate (1.25–2 μM) significantly slows the growth of endothelial cells, while ibandronate (2 μM) also slows the formation of capillary-like tubes and speeds up endothelial cell apoptosis. Ibandronate (< 100 μM) increases VEGF expression in endothelial cells in a dose-dependent manner. [1] In a dose-dependent manner, ibandronate (< 100 μM) inhibits the growth of the two prostate cancer cell lines, LNCaP and PC-3. [2] |
| ln Vivo | Ibandronate significantly lowers the risk of new morphometric vertebral fractures in osteoporotic women after 3 years of treatment when given either daily (2.5 mg) or intermittently (20 mg every other day for 12 doses every 3 months). After three years of treatment, ibandronate significantly and gradually raises the BMD of the lumbar spine in osteoporotic women by 6.5% and 5.7%, respectively, when taken either daily (2.5 mg) or intermittently (20 mg every other day for 12 doses every three months).[3] Ibandronate ( 125 mg/kg s.c.) increases bone mineral density (BMD), trabecular bone volume and number, load to failure (Fmax), and yield load in long bones and vertebrae in ovariectomized rats.Additionally, all doses completely prevent increased trabecular separation in ovariectomized rats. [4] |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Ibandronate is poorly absorbed orally (average in adults 6% on an empty stomach, negligible with food), so absorption of ibandronate by a breastfed infant is unlikely. However, since no information is available on the use of ibandronate during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| References |
[1]. Microvasc Res . 2009 Dec;78(3):453-8. [2]. Acta Oncol . 2011 Jan;50(1):127-33. [3]. J Bone Miner Res . 2004 Aug;19(8):1241-9. [4]. J Rheumatol . 2002 Oct;29(10):2200-8. [5]. Transplantation . 2012 Feb 15;93(3):331-6. |
| Additional Infomation |
Ibandronate Sodium is the sodium salt form of ibandronic acid, a synthetic nitrogen-containing bisphosphonate. Ibandronic acid inhibits farnesyl pyrophosphate synthase, resulting in a reduction in geranylgeranyl GTPase signaling proteins and apoptosis of osteoclasts. This agent increases bone mineral density, decreases bone remodeling, inhibits osteoclast-mediated bone resorption, and reduces metastases-related and corticosteroid-related bone pain. Aminobisphosphonate that is a potent inhibitor of BONE RESORPTION. It is used in the treatment of HYPERCALCEMIA associated with malignancy, for the prevention of fracture and bone complications in patients with breast cancer and bone metastases, and for the treatment and prevention of POSTMENOPAUSAL OSTEOPOROSIS. See also: Ibandronate Sodium (annotation moved to). Drug Indication Treatment of osteoporosis in postmenopausal women at increased risk of fracture (see section 5. 1). A reduction in the risk of vertebral fractures has been demonstrated, efficacy on femoral neck fractures has not been established. Bondronat is indicated for: prevention of skeletal events (pathological fractures, bone complications requiring radiotherapy or surgery) in patients with breast cancer and bone metastases; treatment of tumour-induced hypercalcaemia with or without metastases. Treatment of tumour-induced hypercalcaemia with or without metastases. |
Solubility Data
| Solubility (In Vitro) |
H2O: ~25 mg/mL (~69.6 mM) DMSO: <1 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: 25 mg/mL (69.59 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7838 mL | 13.9190 mL | 27.8381 mL | |
| 5 mM | 0.5568 mL | 2.7838 mL | 5.5676 mL | |
| 10 mM | 0.2784 mL | 1.3919 mL | 2.7838 mL |