IT1t is a novel, potent and selective CXCR4 antagonist with an IC50 of 1.1 nM in calcium mobilization assay, it can be potentially used as an anti-HIV agent.
Physicochemical Properties
| Molecular Formula | C21H36CL2N4S2 |
| Molecular Weight | 479.573340415955 |
| Exact Mass | 478.175 |
| Elemental Analysis | C, 52.60; H, 7.57; Cl, 14.78; N, 11.68; S, 13.37 |
| CAS # | 1092776-63-0 |
| Related CAS # | IT1t; 864677-55-4 |
| PubChem CID | 25178351 |
| Appearance | White to off-white solid powder |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 29 |
| Complexity | 614 |
| Defined Atom Stereocenter Count | 0 |
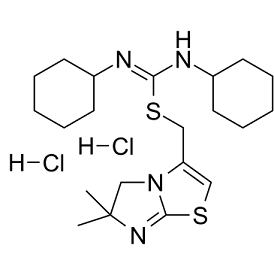
| SMILES | CC1(C)CN2C(CS/C(NC3CCCCC3)=N\C4CCCCC4)=CSC2=N1.[H]Cl.[H]Cl |
| InChi Key | HFXJOXOIINQOEB-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C21H34N4S2.2ClH/c1-21(2)15-25-18(14-27-20(25)24-21)13-26-19(22-16-9-5-3-6-10-16)23-17-11-7-4-8-12-17;;/h14,16-17H,3-13,15H2,1-2H3,(H,22,23);2*1H |
| Chemical Name | (6,6-dimethyl-5H-imidazo[2,1-b][1,3]thiazol-3-yl)methyl N,N'-dicyclohexylcarbamimidothioate;dihydrochloride |
| Synonyms | IT1t dihydrochloride; IT1t; Isothiourea-1t |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | CXCL12/CXCR4 ( IC50 = 2.1 nM ); HIV-1 (X4) ( IC50 = 14.2 nM ); HIV-1 (X4) ( IC50 = 19 nM ) |
| ln Vitro | The CXCR4 plays a role in cancer metastasis, chemotaxis, and acts as a coreceptor for T-tropic HIV-1 viral entry. IT1t is a small isothiourea derivative that resembles a drug. With an IC50 of 2.1 nM, IT1t exhibits very strong and dose-dependent inhibition of the CXCL12/CXCR4 interaction. IT1t likewise inhibits this calcium flux, with an IC50 of 23.1[1]. IT1t exhibits a strong electron density in the binding cavities of the two CXCR4 homodimer subunits. Only the extracellular side of helices V and VI is where monomers in dimers of CXCR4 bound to IT1t interact; the intracellular regions are separated by at least 4 Å, which is likely filled with lipids. Many of the receptor-ligand contacts in the co-crystal structures shown, such as the acidic Asp187, Glu2887.39, and Asp972.63, are critical for CXCL12 binding. The IT1t compound and CVX15 peptide have both been identified as competitive inhibitors of CXCL12. It's possible that the IT1t binding site points to this domain's main anchor region[2]. |
| ln Vivo | IT1t inhibits the zebrafish xenograft model's ability to form TNBC early metastases. Similar to IT1t, the antagonist CXCR4 effectively reduces tumor cell invasion at the metastatic site (Fig. 7B)[3]. |
| Cell Assay | For two hours, serial dilutions of IT1t (0.001, 0.01, 0.1, 1, 10, 100, and 1000 μM) are incubated with Jurkat cells at room temperature. Since these cell types are used in assays for HIV activity that can last up to ten days, the cytotoxicity of IT1t is also assessed at 37°C over a longer duration in MT-4 cells and PHA-stimulated PBMCs (ten day incubation). A kit is used to assess viability and evaluate cytotoxicity under a microscope[1]. |
| References |
[1]. Comparison of cell-based assays for the identification and evaluation of competitive CXCR4 inhibitors. PLoS One. 2017 Apr 14;12(4):e0176057. [2]. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010 Nov 19;330(6007):1066-71. [3]. Inhibition of signaling between human CXCR4 and zebrafish ligands by the small molecule IT1timpairs the formation of triple-negative breast cancer early metastases in a zebrafish xenograft model. Dis Model Mech. 2016 Feb;9(2):141-533. |
Solubility Data
| Solubility (In Vitro) |
DMSO: ≥ 30 mg/mL (~62.6 mM) H2O: ~50 mg/mL (~104.3 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.67 mg/mL (3.48 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1.67 mg/mL (3.48 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 1.67 mg/mL (3.48 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 100 mg/mL (208.52 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0852 mL | 10.4260 mL | 20.8520 mL | |
| 5 mM | 0.4170 mL | 2.0852 mL | 4.1704 mL | |
| 10 mM | 0.2085 mL | 1.0426 mL | 2.0852 mL |