Physicochemical Properties
| Molecular Formula | C23H29NO12 |
| Molecular Weight | 511.48 |
| Exact Mass | 511.169 |
| Elemental Analysis | C, 54.01; H, 5.72; N, 2.74; O, 37.54 |
| CAS # | 6379-56-2 |
| PubChem CID | 6433481 |
| Appearance | Typically exists as White to light yellow solid at room temperature |
| Source | Streptomyces hygroscopicus |
| LogP | -1.8 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 36 |
| Complexity | 853 |
| Defined Atom Stereocenter Count | 10 |
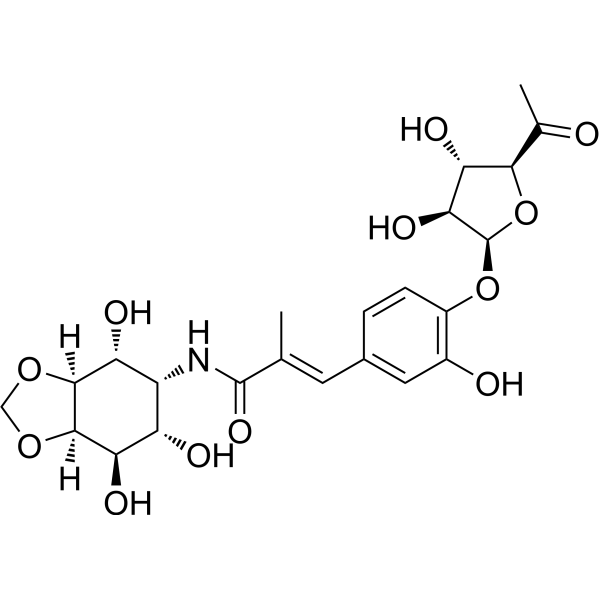
| SMILES | CC(=CC1=CC(=C(C=C1)OC2C(C(C(O2)C(=O)C)O)O)O)C(=O)NC3C(C(C4C(C3O)OCO4)O)O |
| InChi Key | YQYJSBFKSSDGFO-IIHALWDASA-N |
| InChi Code | InChI=1S/C23H29NO12/c1-8(22(32)24-13-14(27)16(29)21-20(15(13)28)33-7-34-21)5-10-3-4-12(11(26)6-10)35-23-18(31)17(30)19(36-23)9(2)25/h3-6,13-21,23,26-31H,7H2,1-2H3,(H,24,32)/b8-5+/t13-,14+,15-,16-,17+,18+,19-,20+,21-,23-/m1/s1 |
| Chemical Name | (E)-N-[(3aS,4R,5R,6S,7R,7aR)-4,6,7-trihydroxy-3a,4,5,6,7,7a-hexahydro-1,3-benzodioxol-5-yl]-3-[4-[(2S,3S,4S,5S)-5-acetyl-3,4-dihydroxyoxolan-2-yl]oxy-3-hydroxyphenyl]-2-methylprop-2-enamide |
| Synonyms | Hygromycin A; 6379-56-2; HYGROMYCIN; Homomycin; UNII-3YJY415DDI; HYGROMYCIN [MI]; (E)-N-[(3aS,4R,5R,6S,7R,7aR)-4,6,7-trihydroxy-3a,4,5,6,7,7a-hexahydro-1,3-benzodioxol-5-yl]-3-[4-[(2S,3S,4S,5S)-5-acetyl-3,4-dihydroxyoxolan-2-yl]oxy-3-hydroxyphenyl]-2-methylprop-2-enamide; 3YJY415DDI; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Antibiotic |
| ln Vitro | Lyme disease, which is caused by the spirochete Borrelia burgdorferi, is on the rise. Current treatment relies on broad-spectrum antibiotics that perturb the gut microbiome. In a recent paper in Cell, Leimer et al. demonstrate the utility of a long-forgotten antibiotic, Hygromycin A, as a spirochete-specific antibacterial that is conducive to gut health[1]. |
| ln Vivo | Hygromycin A produces probiotics by killing Borrelia burgdorferi selectively and improving the makeup of the gut flora [1]. |
| Cell Assay |
In a recent Cell paper, Leimer et al., (2021) address the lack of a specific and/or selective antibiotic for B. burgdorferi with the surprising rediscovery of Hygromycin A, which was first discovered in 1953 and later forgotten due to its poor potency against most Gram-positive and Gram-negative organisms (Pittenger et al., 1953). Traditional screens for antibiotics are focused on compounds that present a broad-spectrum -cidal activity against multiple bacteria. Using a non-conventional approach, the authors instead focused on compounds with a selective-cidal activity against B. burgdorferi, and so they identified Hygromycin A, which is produced copiously by the soil bacterium Streptomyces hygroscopicus. They found that Hygromycin A was also effective against genospecies of Lyme-disease-causing spirochetes found in Eurasia, B. garinii, and B. afzeli. Adding to the importance of this study is the finding that Hygromycin A is also effective against Treponema pallidum, the agent of syphilis. This could be highly significant because, unlike those for Lyme disease, an increasing level of antibiotic resistance to some of the antibiotics used to treat syphilis is being observed (Stamm, 2010). [1] The authors elegantly and systematically unravel a mechanistic understanding of the specificity of Hygromycin A for B. burgdorferi (and other spirochetes). Hygromycin A binds to 23S rRNA of the 70S subunit of the bacterial ribosome, which forms the core of the peptidyl transferase center, and effectively impairs protein synthesis (Polikanov et al., 2015). Given that the 23S rRNA sequence is highly conserved across bacterial species, why is the antibiotic so ineffective against most pathogens? Hydrophilic molecules such as Hygromycin A are normally prevented from entering bacterial cells due to the hydrophobic nature of the cell membrane barrier. Gram-negative bacteria have an inner and outer membrane surrounding the cell wall, while Gram-positive bacteria only have an inner membrane. Transmembrane multidrug efflux pumps also help to extrude any antibiotic that might breach the barrier (Peterson and Kaur, 2018). Using efflux and porin mutants of Escherichia coli, the authors demonstrate that Hygromycin A is excluded from entering Gram-negative and Gram-positive bacteria predominantly by the cytoplasmic membrane barrier. Although B. burgdorferi lacks typical lipopolysaccharide (LPS) that is found in other Gram-negative bacteria, it does retain an outer and inner membrane barrier. Unlike many other Gram-negative bacteria, B. burgdorferi lacks the enzymes for de novo synthesis of purines—quintessential building blocks of RNA and DNA. Instead, it encodes and is dependent upon a nucleoside transporter, Basic membrane protein D (BmpD), which is a periplasmic substrate binding protein and is part of the ATP-binding cassette (ABC)-type purine nucleoside transporter that functions to scavenge purine nucleosides from the host environment (Cuellar et al., 2020). Hygromycin A has a structural resemblance to a purine nucleoside, and it gets a free ride into the spirochete via BmpD, allowing it to preferentially halt protein synthesis in spirochetes (Figure 1). The study provides compelling evidence, using robust molecular tools, that BmpD—along with BmpD orthologs in other spirochetes—is the basis for the unique transport of Hygromycin A. This explains the selectivity of Hygromycin A for spirochetes.[1] |
| Toxicity/Toxicokinetics |
mouse LD50 intraperitoneal 1067 mg/kg Index of Antibiotics from Actinomycetes, Umezawa, H. et al., eds., Tokyo, Univ. of Tokyo Press, 1967, -(656), 1967 mouse LD50 intravenous 200 mg/kg CRC Handbook of Antibiotic Compounds, Vols.1- , Berdy, J., Boca Raton, FL, CRC Press, 1980, 6(332), 1981 |
| References | [1]. Hygromycin A in the Lymelight. Cell Host Microbe. 2021 Nov 10;29(11):1599-1601. |
| Additional Infomation |
Hygromycin A is a hydroxycinnamic acid. It has a role as a metabolite. Hygromycin A has been reported in Streptomyces, Streptomyces noboritoensis, and other organisms with data available. This work sets the stage for preclinical studies on efficacy, toxicity, and bioavailability in the further development of Hygromycin A as a selective treatment modality for Lyme disease. The authors suggest that oral delivery of Hygromycin A to mice results in a significantly favorable effect on the gut microbiome composition (Figure 1). The possible effects of this antibiotic on the human microbiome composition remain to be seen. Changes in the microbiome composition have been associated with antibiotic refractory Lyme arthritis and post-treatment Lyme disease syndrome (sometimes referred to as chronic Lyme disease) (Morrissette et al., 2020). Whether these changes are a cause or an effect in these hotly debated clinical manifestations is not understood; however, it is always beneficial to replace a broad-spectrum antibiotic with an effective selective one wherever possible. It is important to note that although the use of Hygromycin A may limit broad impacts on the microbiome, selective uptake will limit its efficacy to B. burgdorferi, and unlike doxycycline, it will not be efficacious in the treatment of other possible coinfecting tick-borne bacterial pathogens. The authors also suggest that the possible utility of the antibiotic extends to controlling B. burgdorferi prevalence in reservoir hosts and in ticks through the use of baits loaded with Hygromycin A (Figure 1). They hypothesize that both the specificity of Hygromycin A and the fact that B. burgdorferi carries just one copy of the BmpD gene, which is required for growth, makes it less likely that there would be a rapid spread of antibiotic resistance among the spirochetes in the reservoir hosts. However, we would caution that this claim has been made about other antibiotics in the past, only to have resistance develop eventually. It is important to note here that antimicrobial resistance (AMR) has not been an issue in Lyme disease because humans are a dead-end host for Borrelia. Thus, even if an antibiotic-resistant Borrelia strain were to develop in an individual human, it would not spread beyond that individual. However, employing antibiotics used to treat human disease to also treat reservoir hosts is likely to foster the spread of antimicrobial-resistant strains of Borrelia, and this could eventually impact treatment efficacy in humans. Despite these caveats, the study brings to center stage a potentially viable alternative to treat Lyme disease and possibly other spirochetal diseases.[1] |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~195.51 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9551 mL | 9.7756 mL | 19.5511 mL | |
| 5 mM | 0.3910 mL | 1.9551 mL | 3.9102 mL | |
| 10 mM | 0.1955 mL | 0.9776 mL | 1.9551 mL |