|
Histamine (phosphate) is a strong histamine receptor agonist/activator and vasodilator neuroagent, capable of activating nitric oxide synthase.
|
Physicochemical Properties
| Molecular Formula |
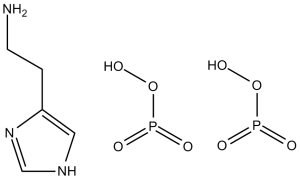
C5H15N3O8P2
|
| Molecular Weight |
307.14
|
| Exact Mass |
307.03
|
| Elemental Analysis |
C, 19.55; H, 4.92; N, 13.68; O, 41.67; P, 20.17
|
| CAS # |
51-74-1
|
| Related CAS # |
Histamine dihydrochloride; 56-92-8; Histamine; 51-45-6
|
| PubChem CID |
65513
|
| Appearance |
White to off-white solid powder
|
| Boiling Point |
887.3ºC at 760 mmHg
|
| Melting Point |
128-132 °C
|
| Flash Point |
490.4ºC
|
| Hydrogen Bond Donor Count |
8
|
| Hydrogen Bond Acceptor Count |
10
|
| Rotatable Bond Count |
2
|
| Heavy Atom Count |
18
|
| Complexity |
115
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
NCCC1=CNC=N1.O=P(O)(O)O.O=P(O)(O)O
|
| InChi Key |
ZHIBQGJKHVBLJJ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C5H9N3.2H3O4P/c6-2-1-5-3-7-4-8-5;2*1-5(2,3)4/h3-4H,1-2,6H2,(H,7,8);2*(H3,1,2,3,4)
|
| Chemical Name |
2-(1H-imidazol-5-yl)ethanamine;phosphoric acid
|
| Synonyms |
Histamine acid phosphate; Histamine diphosphate; Histamine phosphate; Histamine biphosphate; Histamine dihydrogen phosphate; Histamine diphosphate; 51-74-1; Histamine acid phosphate; Histamine biphosphate; 2-(1H-imidazol-4-yl)ethanamine bis(phosphate); Histamine phosphate (1:2); Histamine dihydrogen phosphate;
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder-20°C 3 years
4°C 2 years
In solvent -80°C 6 months
-20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture.
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
|
Biological Activity
| Targets |
Histamine H1 receptor; Histamine H2 receptor
|
| ln Vitro |
In vitro activity: Histamine (10 μM) results in a greater build-up of inositol monophosphate in the chromaffin cells of the cow adrenal gland. The amount of radioactivity in the InsP3-containing fraction of bovine adrenal chromaffin cells is stimulated by histamine (10 μM). Compared to angiotensin I1 and bradykinin, histamine (100 μM) stimulates incorporation into the InsP3-containing eluate less. [1] |
|
| ln Vivo |
| Histamine phosphate (0.025 mg/kg) produces a mean increase in basilar blood flow of 145% of control in dogs. Histamine phosphate produces considerable increases in basilar blood flow as well as a decrease in femoral arterial blood pressure in dogs when injected intravenously and measured with an electromagnetic flow transducer. [2] In sheep that are not anesthetized, histamine phosphate (4 μg/kg) causes lymph flow to increase from 6.0 to 27.0 (SEM) ml/h. In addition, unanesthetized sheep treated with histamine phosphate (4 μg/kg) had decreases in cardiac output and arterial PO2 and increases in lung water, pulmonary vascular resistance, arterial PCO2, pH, and hematocrit. [3] In dogs that are anesthetized open-chested, histamine phosphate (8.3 mg/kg/min) has no discernible effect on pulmonary lymph flow (QL) or protein concentration (CL); nevertheless, both increase following alloxan. In anesthetized dogs with open chests, histamine phosphate (8.3 mg/kg/min) also has no discernible effect on the maximum capillary pressure (PCcritical) or the pulmonary capillary membrane filtration coefficient (Kf). [4] In the unanesthetized intact rat, histamine phosphate (50 mg/kg) causes a noticeable increase in acid secretion, but does not affect pepsin output. In the unanesthetized intact rat, histamine phosphate (50 mg/kg) stimulates gastric acid secretion to the greatest extent possible without causing any harmful side effects. [5] |
|
| Cell Assay |
Histamine, bradykinin, and angiotensin II stimulate release of catecholamines from adrenal medulla. Here we show, using bovine adrenal chromaffin cells in culture, that these agonists as well as carbachol (with hexamethonium) stimulate production of inositol phosphates. The histamine response was mepyramine sensitive, implicating an H1 receptor, whereas bradykinin had a lower EC50 than Met-Lys-bradykinin, and [Des-Arg9]-bradykinin was relatively inactive, implicating a BK-2 receptor. Total inositol phosphates formed in the presence of lithium were measured, with histamine giving the largest response. The relative contribution of chromaffin cells and nonchromaffin cells in the responses was assessed. In each case chromaffin cells were found to be responding to the agonists; in the case of histamine the response was solely on chromaffin cells. When the inositol phosphates accumulating over 2 or 5 min, with no lithium present, were separated on Dowex anion-exchange columns, bradykinin gave the greatest stimulation in the inositol trisphosphate fraction, whereas histamine gave a larger inositol monophosphate accumulation. On resolution of the isomers of stimulated inositol trisphosphate after 2 min of stimulation, the principal isomer present was inositol 1,3,4-trisphosphate in each case. Two hypotheses for the differential responses to histamine and bradykinin are discussed[1].
|
| Animal Protocol |
| 8.3 mg/kg | | Rat |
An intermandibular-transclival approach to the posterior cranial fossa has been developed which allows exposure of the basilar artery for attachment of a small electromagnetic blood flow transducer. The results of single intravenous injections of betahistine hydrochloride indicated a mean increase in basilar artery blood flow of 54% and a simultaneous decrease in systemic arterial blood pressure of a duration of action of approximately one minute. Histamine phosphate yielded results similar to betahistine hydrochloride, while nicotinic acid produced only slight increases in blood flow in the basilar artery.[2]
To see whether antihistamines could prevent and reverse histamine-induced pulmonary edema and increased lung vascular permeability, we compared the effects of a 4-h intravenous infusion of 4 mug/kg per min histamine phosphate on pulmonary hemodynamics, lung lymph flow, lymph and plasma protein content, arterial blood gases, hematocrit, and lung water with the effects of an identical histamine infusion given during an infusion of diphenhydramine or metiamide on the same variables in unanesthetized sheep. Histamine caused lymph flow to increase from 6.0+/-0.5 to 27.0+/-5.5 (SEM) ml/h (P less than 0.05), lymph; plasma globulin concentration ratio to increase from 0.62+/-0.01 to 0.67+/-0.02 (P less than 0.05), left atrial pressure to fall from 1+/-1 to -3+/-1 cm H2O (P less than 0.05), and lung lymph clearance of eight protein fractions ranging from 36 to 96 A molecular radius to increase significantly. Histamine also caused increases in lung water, pulmonary vascular resistance, arterial PCO2, pH, and hematocrit, and decreases in cardiac output and arterial PO2. Diphenhydramine (3 mg/kg before histamine followed by 1.5 mg/kg per h intravenous infusion) completely prevented the histamine effect on hematocrit, lung lymph flow, lymph protein clearance, and lung water content, and reduced histamine effects on arterial blood gases and pH. 6 mg/kg diphenhydramine given at the peak histamine response caused lymph flow and lymph: plasma protein concentration ratios to fall. Metiamide (10 mg/kg per h) did not affect the histamine lymph response. We conclude that diphenhydramine can prevent histamine-induced pulmonary edema and can prevent and reverse increased lung vascular permeability caused by histamine, and that histamine effects on lung vascular permeability are H1 actions.[3]
We estimated the pulmonary capillary membrane filtration coefficient (Kf) and the maximum capillary pressure (PCcritical) at which the lung could maintain a constant weight in 1) 5 control experiments in anesthetized open-chested dogs, 2) 7 experiments in which the dogs were given 3.6-8.3 microgram . kg-1 . min-1 of histamine phosphate, and 3) in 6 experiments after 75-100 mg/kg of alloxan. In additional experiments, pulmonary lymph flow (QL) and protein concentration (CL) were measured during the infusion of histamine and alloxan. After histamine, Kf averaged 0.045 +/- 0,008 ml . min-1mmHg-1 (SE) and PCcritical was 22.1 +/- 1.1 mmHg. These values were not significantly different from the control Kf and PCcritical (0.036 +/- 0.006 and 22.5 +/- 2.3, respectively). After alloxan, Kf (1.43 +/- 0.69) was larger and PCcritical (12.4 +/- 1.3) was significantly less than control (P less than 0.05). Histamine caused no significant change in QL or CL; however, both were increased after alloxan. These results show that Kf, PCcritical, QL, and CL are all changed by an increase in capillary membrane permeability caused by alloxan. Because none of these factors as significantly affected by histamine, dog lung capillary membrane permeability may not be affected by histamine.[4]
|
| References |
[1]. J Neurochem
. 1988 Aug;51(2):634-41.
[2]. Stroke
. 1971 Jul-Aug;2(4):409-15.
[3]. J Clin Invest
. 1976 Aug;58(2):391-8.
[4]. Am J Physiol
. 1980 Jul;239(1):H96-100.
[5]. Gut
. 1961 Mar;2(1):32-6.
|
| Additional Infomation |
Histamine phosphate is a phosphate salt that is the diphosphate salt of histamine. It has a role as a histamine agonist. It contains a histamine.
See also: Histamine (has active moiety).
|
|
Solubility Data
| Solubility (In Vitro) |
| DMSO: ~1.4 mg/mL (4.7 mM) | | Water: ~42 mg/mL (~136.7 mM) | | Ethanol: <1 mg/mL |
|
| Solubility (In Vivo) |
Solubility in Formulation 1: 50 mg/mL (162.79 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication.
(Please use freshly prepared in vivo formulations for optimal results.)
|
| Preparing Stock Solutions |
|
1 mg |
5 mg |
10 mg |
| 1 mM |
3.2558 mL |
16.2792 mL |
32.5584 mL |
| 5 mM |
0.6512 mL |
3.2558 mL |
6.5117 mL |
| 10 mM |
0.3256 mL |
1.6279 mL |
3.2558 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles. |