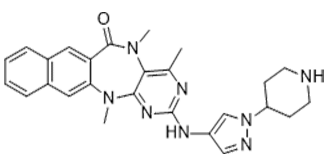HTH-01-015 is a novel, potent and selective inhibitor of NUAK1( (NUAK family SnF1-like kinase-1) which can serve as useful chemical probes to delineate the biological roles of the NUAK kinases. It inhibits NUAK1 with an IC50 of less than 100 nM and has an IC50 that is >100 -fold higher for NUAK1 than for NUAK2 (IC50 of >10 μM). The LKB1 tumour suppressor kinase activates the NUAK1 and NUAK2 protein kinases, which are members of the AMPK (AMP-activated protein kinase) family.
Physicochemical Properties
| Molecular Formula | C26H28N8O |
| Molecular Weight | 468.55 |
| Exact Mass | 468.238 |
| Elemental Analysis | C, 66.65; H, 6.02; N, 23.91; O, 3.41 |
| CAS # | 1613724-42-7 |
| Related CAS # | 1613724-42-7 |
| PubChem CID | 78357766 |
| Appearance | Light yellow to khaki solid powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 759.6±70.0 °C at 760 mmHg |
| Flash Point | 413.2±35.7 °C |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.750 |
| LogP | 1.78 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 35 |
| Complexity | 762 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=C1C2=C([H])C3=C([H])C([H])=C([H])C([H])=C3C([H])=C2N(C([H])([H])[H])C2C(=C(C([H])([H])[H])N=C(N=2)N([H])C2C([H])=NN(C=2[H])C2([H])C([H])([H])C([H])([H])N([H])C([H])([H])C2([H])[H])N1C([H])([H])[H] |
| InChi Key | CHSDJDLAKKAWCI-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C26H28N8O/c1-16-23-24(31-26(29-16)30-19-14-28-34(15-19)20-8-10-27-11-9-20)32(2)22-13-18-7-5-4-6-17(18)12-21(22)25(35)33(23)3/h4-7,12-15,20,27H,8-11H2,1-3H3,(H,29,30,31) |
| Chemical Name | 2,7,9-trimethyl-5-[(1-piperidin-4-ylpyrazol-4-yl)amino]-2,4,6,9-tetrazatetracyclo[9.8.0.03,8.013,18]nonadeca-1(19),3,5,7,11,13,15,17-octaen-10-one |
| Synonyms | HTH-01015; HTH01015; HTH 01015; HTH-01-015; HTH01-015; HTH 01-015 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | NUAK1 (IC50 = 100 nM) |
| ln Vitro | In a 24-well plate, 2.5 105 HsSultan or NB4 cells are plated in 500 L of phenol red-free RPMI medium with 10% FBS. Each compound (8 µgHTH-01-015 inhibits NUAK1-mediated phosphorylation of MYPT1 in HEK-293 cells that express both NUAK1 and NUAK2. In NUAK1+/+ MEFs, HTH-01-015 inhibits U2OS cell invasion and cell migration. In addition, HTH-01-015 prevents cell division in both cell lines.[1] In U2OS cells, HTH-01-015 inhibitors significantly reduced the number of cells that could undergo mitosis.[2] |
| ln Vivo | Short-term treatment of normal Sprague Dawley rats with A-769662 decreases liver malonyl CoA levels and the respiratory exchange ratio, VCO2/VO2, indicating an increased rate of whole-body fatty acid oxidation. Treatment of ob/ob mice with 30 mg/kg b.i.d. A-769662 decreases hepatic expression of PEPCK, G6Pase, and FAS, lowers plasma glucose by 40%, reduced body weight gain and significantly decreases both plasma and liver triglyceride levels. |
| Enzyme Assay | In HEK-293 cells express NUAK1 as well as NUAK2, HTH-01-015 suppresses NUAK1-mediated MYPT1 phosphorylation. In NUAK1+/+ MEFs, HTH-01-015 inhibits U2OS cell invasion and cell migration. In addition, HTH-01-015 prevents cell division in both cell lines. [1] In U2OS cells, HTH-01-015 inhibitors significantly reduced the number of cells that could undergo mitosis. The incorporation of radioactive 32P from [-32P]ATP into the Sakamototide substrate peptide is measured using Cerenkov counting to determine the in vitro activities of purified GST-NUAK1 and GST-NUAK1[A195T]. In order to stop a reaction, 40 mL of the reaction mixture must be spotted onto P81 paper and immediately submerged in 50 mM orthophosphoric acid. Reactions are conducted in a 50 μL reaction volume for 30 min at 30°C. |
| Cell Assay | Cell proliferation assays are carried out colorimetrically in 96-well plates using the CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay kit following the manufacturer's protocol. U2OS cells and MEFs are initially seeded at a rate of 2000 and 3000 cells, respectively, per well. Five days are spent performing the proliferation assays with or without 10 M HTH-01-015. |
| Animal Protocol | NA; |
| References |
[1]. Biochem J . 2014 Jan 1;457(1):215-25. [2]. Biochem J . 2014 Jul 15;461(2):233-45. |
Solubility Data
| Solubility (In Vitro) |
DMSO: ~58 mg/mL (~123.8 mM) Water: <1 mg/mL Ethanol: 30 mg/mL (~64.0 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.34 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.34 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.34 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1342 mL | 10.6712 mL | 21.3424 mL | |
| 5 mM | 0.4268 mL | 2.1342 mL | 4.2685 mL | |
| 10 mM | 0.2134 mL | 1.0671 mL | 2.1342 mL |