Grazoprevir (formerly MK-5172; MK5172; trade name: Zepatier) is a 2nd generation and selective inhibitor of the Hepatitis C Virus NS3/4A Protease approved by FDA in 2016 for the treatment of hepatitis C (used in combination with elbasvir, an NS5A replication complex inhibitor). It inhibits HCV genotype 1a, 1B, and 4 with IC50 values of 7pM, 4pM, and 62pM, respectively. Grazoprevir has a broad spectrum of anti-HCV activity against various genotypes and resistant variants.
Physicochemical Properties
| Molecular Formula | C38H50N6O9S |
| Molecular Weight | 766.9 |
| Exact Mass | 766.335 |
| Elemental Analysis | C, 59.51; H, 6.57; N, 10.96; O, 18.78; S, 4.18 |
| CAS # | 1350514-68-9 |
| Related CAS # | Grazoprevir potassium salt;1206524-86-8;Grazoprevir hydrate;1350462-55-3;Grazoprevir sodium salt;1425038-27-2 |
| PubChem CID | 44603531 |
| Appearance | White to off-white solid powder |
| Density | 1.4±0.1 g/cm3 |
| Index of Refraction | 1.633 |
| LogP | 3.93 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 8 |
| Heavy Atom Count | 54 |
| Complexity | 1580 |
| Defined Atom Stereocenter Count | 7 |
| SMILES | S(C1([H])C([H])([H])C1([H])[H])(N([H])C([C@]1(C([H])([H])[C@@]1([H])C([H])=C([H])[H])N([H])C([C@]1([H])C([H])([H])[C@]2([H])C([H])([H])N1C([C@]([H])(C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])N([H])C(=O)O[C@]1([H])C([H])([H])[C@@]1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1C(=NC3C([H])=C(C([H])=C([H])C=3N=1)OC([H])([H])[H])O2)=O)=O)=O)(=O)=O |
| InChi Key | OBMNJSNZOWALQB-NCQNOWPTSA-N |
| InChi Code | InChI=1S/C38H50N6O9S/c1-6-22-19-38(22,35(47)43-54(49,50)25-13-14-25)42-32(45)29-18-24-20-44(29)34(46)31(37(2,3)4)41-36(48)53-30-16-21(30)10-8-7-9-11-27-33(52-24)40-28-17-23(51-5)12-15-26(28)39-27/h6,12,15,17,21-22,24-25,29-31H,1,7-11,13-14,16,18-20H2,2-5H3,(H,41,48)(H,42,45)(H,43,47)/t21-,22-,24-,29+,30-,31-,38-/m1/s1 |
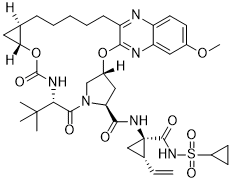
| Chemical Name | (33R,35S,91R,92R,5S)-5-(tert-butyl)-N-((1R,2S)-1-((cyclopropylsulfonyl)carbamoyl)-2-vinylcyclopropyl)-17-methoxy-4,7-dioxo-2,8-dioxa-6-aza-1(2,3)-quinoxalina-3(3,1)-pyrrolidina-9(1,2)-cyclopropanacyclotetradecaphane-35-carboxamide |
| Synonyms | MK5172; MK 5172; MK-5172; Trade name: Zepatier; 1350514-68-9; MK-5172; Grazoprevir anhydrous; MK5172; Grazoprevir [INN]; MK-5172 ANHYDROUS; 8YE81R1X1J; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | gt1b(Ki=0.01±<0.01 nM);gt1a(Ki=0.01±0.01 nM);gt2a(Ki=0.08±0.02 nM);gt2b(Ki=0.15±0.06 nM);gt3a(Ki=0.90±0.2 nM) |
| ln Vitro | MK-5172 (Grazoprevir) is effective in biochemical assays against major genotypes and variants engineered with common resistant mutations, with Ki of 0.01±<0.01 nM (gt1b), 0.01±0.01 nM (gt1a), 0.08±0.02 nM (gt2a), 0.15±0.06 nM (gt2b), 0.90±0.2 nM (gt3a), 0.07±0.01 nM (gt1bR155K), 0.14±0.03 nM (gt1bD168V), 0.30±0.04 nM (gt1bD168Y), 5.3±0.9 nM (gt1bA156T), and 12±2 nM (gt1bA156V), respectively. In the replicon assay, MK-5172 demonstrates subnanomolar to low-nanomolar EC50s against genotypes 1a, 1b, and 2a, with EC50s of 0.5±0.1 nM, 2±1 nM, and 2±1 nM for gt1bcon1, gt1a, and gt2a, respectively. MK-5172 is potent against a panel of HCV replication mutants NS5A (Y93H) (EC50=0.7±0.3 nM), NS5B nucleosides (S282T) (EC50=0.3±0.1 nM), and NS5B (C316Y) (EC50=0.4±0.2). MK-5172 maintains the excellent potency against the gt 3a enzyme as well as a broad panel of mutant enzymes, has excellent potency in the replicon system [gt1b IC50(50% NHS)=7.4 nM; gt1a IC50(40% NHS)=7 nM], and shows excellent rat liver exposure. |
| ln Vivo | Against chimpanzees with chronic HCV infection, MK-5172 (grazoprevir) exhibits a high level of in vivo efficacy. When administered intravenously to dogs, MK-5172 exhibits a low clearance of 5 mL/min/kg and a 3-hour half-life. Following an oral dose of 1 mg/kg, it has a good plasma exposure (AUC=0.4 μM h). Following an oral dose of 1 mg/kg, studies on dog liver biopsy revealed that the liver concentration of MK-5172 was 1.4 μM at the 24-hour mark. Twenty-four hours after oral dosing in dogs, MK-5172 exhibits good liver tissue partitioning and maintains high liver concentration relative to potency, which is consistent with its behavior in rats. |
| Enzyme Assay | recombinant HCV NS3/4A enzymes are expressed and purified from E. Coli. Enzyme sequences are derived from genotype 1a (gt1a) H77, gt1b con1, gt2a JFH1, gt2b HCJ8, and gt3a NZL1. Inhibition of HCV NS3/4A protease activity in reaction mixtures containing MK-5172 (Grazoprevir), Vaniprevir, or the reference compounds Danoprevir and TMC435 is determined in a time-resolved fluorescence assay. Cell-based HCV replicon assays are conducted in genotype 1b (con1) stable cell line HB1 or a gt2a cell line (JFH) in the presence of either 10% fetal bovine serum (FBS) or 40% normal human serum (NHS). Determinations of 50% effective concentrations (EC50s) against the panel of genotype or mutant replicon cell lines are conducted using a TaqMan-based assay. The 50% cytotoxic concentration (CC50) is determined in the HCV replicon cell line with the use of an MTS assay. Potency determinations against clinical genotype 1 NS3/4A sequences are made using a transient cell-based phenotype assay. The NS3/4A patient isolates are cloned from human plasma infected with HCV. Broad counterscreening, in which MK-5172 is evaluated for its inhibitory potency at a concentration of 10 μM, is conducted at MDS Pharma Services. |
| Cell Assay | HB1 cells (30,000 per well) are seeded of a 6-well tissue culture plate per drug concentration. The next day (day 0), the medium is replenished with fresh medium and MK-5172 at the appropriate drug concentration. Cells from a single well per drug concentration are harvested on days 0, 1, and 2, washed, and stored frozen until evaluation. The fourth well is similarly harvested on day 3.5 except that 30,000 cells are reseeded with fresh medium and MK-5172 at the appropriate drug concentration. For additional time points, cells are passaged and harvested every one-half week for 2 weeks. For the third week, cells are similarly treated except that cells received replenishing medium which contained 0.5 mg/ml G418 without protease inhibitor. |
| Animal Protocol |
Rats and Dogs Research is conducted on rats and dogs. Grazoprevir is formulated in polyethylene glycol 200 (PEG200) and given as a bolus at either 2 mg/kg of body weight (for rats) or 0.5 mg/kg (for dogs) in studies where the drug is dosed intravenously. The compound's crystalline potassium salt is dosed as a solution in PEG400 at 5 mg/kg (for rats) or 1 mg/kg (for dogs) for oral studies.In every study, blood samples are taken at the appropriate times in tubes containing EDTA, and the plasma is separated by centrifugation and kept at -70°C until analysis. After protein precipitation, Grazoprevir (MK-5172) levels are quantified using high-performance liquid chromatography/mass spectroscopy (LC/MS/MS). At the end of the experiment, liver samples are taken from rat studies. After sedation, liver biopsy samples (20 μL) are taken from the dog. Following protein precipitation, tissue samples are homogenized in four volumes of deionized water, and drug concentrations are assessed using LC/MS/MS. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Grazoprevir reaches peak plasma concentration 0.5-3 hours after administration. Grazoprevir has an absolute bioavailability of 27%. When taken with food the peak concentration of Grazoprevir increases 2.8 fold but this increase in exposure has not been deemed clinically relevant. Grazoprevir is mainly eliminated in the feces (90%) with very little eliminated in the urine (<1%). Grazoprevir has an estimated apparent volume of distribution of 1250 liters. It is thought to distribute primarily to the liver with its uptake facilitated by organic anion transporting polypeptide 1B1/3. The clearance of Grazoprevir has not been determined. Metabolism / Metabolites Grazoprevir is partially eliminated by oxidative metabolism meditated by CYP3A. No circulating metabolites of have been detected in human plasma. Biological Half-Life The geometric mean apparent terminal half-life for Grazoprevir is 31 hours in HCV-infected subjects. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Grazoprevir has not been studied in nursing mothers being treated for hepatitis C infection. Because it is greater than 98.9% bound to maternal plasma proteins, amounts in breastmilk are likely to be very low. Some sources recommend against breastfeeding when grazoprevir is used with ribavirin. Hepatitis C is not transmitted through breastmilk and breastmilk has been shown to inactivate hepatitis C virus (HCV). However, the Centers for Disease Control recommends that mothers with HCV infection should consider abstaining from breastfeeding if their nipples are cracked or bleeding. It is not clear if this warning would apply to mothers who are being treated for hepatitis C. Infants born to mothers with HCV infection should be tested for HCV infection; because maternal antibody is present for the first 18 months of life and before the infant mounts an immunologic response, nucleic acid testing is recommended. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Grazoprevir is more than 98.8% bound to plasma proteins. It binds both human serum albumin and α1-acid glycoprotein. |
| References |
[1]. MK-5172, a selective inhibitor of hepatitis C virus NS3/4a protease with broad activity across genotypes and resistant variants. Antimicrob Agents Chemother. 2012 Aug;56(8):4161-7. [2]. Discovery of MK-5172, a Macrocyclic Hepatitis C Virus NS3/4a Protease Inhibitor. ACS Med Chem Lett. 2012 Mar 2;3(4):332-6. [3]. Bardoxolone and bardoxolone methyl, two Nrf2 activators in clinical trials, inhibit SARS-CoV-2 replication and its 3C-like protease. Signal Transduct Target Ther. 2021 May 29;6(1):212. |
| Additional Infomation |
Grazoprevir is an azamacrocyclic compound that is a hepatitis C protease inhibitor used in combination with elbasvir (under the brand name Zepatier) for treatment of chronic HCV genotypes 1 or 4 infection in adults. It has a role as an antiviral drug, a hepatoprotective agent and a hepatitis C protease inhibitor. It is an azamacrocycle, a carbamate ester, a lactam, an aromatic ether, a member of cyclopropanes, a N-sulfonylcarboxamide and a quinoxaline derivative. Grazoprevir is a direct acting antiviral medication used as part of combination therapy to treat chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV). HCV is a single-stranded RNA virus that is categorized into nine distinct genotypes, with genotype 1 being the most common in the United States, and affecting 72% of all chronic HCV patients. Treatment options for chronic Hepatitis C have advanced significantly since 2011, with the development of Direct Acting Antivirals (DAAs) such as Grazoprevir. Grazoprevir is an inhibitor of NS3/4A, a serine protease enzyme, encoded by HCV genotypes 1 and 4 [synthesis]. These enzymes are essential for viral replication and serve to cleave the virally encoded polyprotein into mature proteins like NS3, NS4A, NS4B, NS5A and NS5B. The barrier for develoment of resistance to NS3/4A inhibitors is lower than that of NS5B inhibitors, another class of DAAs. Subtitutions at amino acid positions 155, 156, or 168 are known to confer resistance. The substitutions of the enzyme's catalytic triad consisting of H58, D82, and S139 are also likely to alter the affinity of the drug for NS3/4A or the activity of the enzyme itself. Despite this disadvantage Grazoprevir is still effective against HCV particularly when paired with [DB11574]. In a joint recommendation published in 2016, the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) recommend Grazoprevir as first line therapy in combination with [DB11574] for genotypes 1a, 1b, and 4 of Hepatitis C. Grazoprevir and [DB11574] are used with or without [DB00811] with the intent to cure, or achieve a sustained virologic response (SVR), after 12 weeks of daily therapy. SVR and eradication of HCV infection is associated with significant long-term health benefits including reduced liver-related damage, improved quality of life, reduced incidence of Hepatocellular Carcinoma, and reduced all-cause mortality. Grazoprevir is available as a fixed dose combination product with [DB11574] (tradename Zepatier) used for the treatment of chronic Hepatitis C. Approved in January 2016 by the FDA, Zepatier is indicated for the treatment of HCV genotypes 1 and 4 with or without [DB00811] depending on the the presence of resistance associated amino acid substitutions in the NS5A protein and previous treatment failure with [DB00811], [DB00008], [DB00022], or other NS3/4A inhibitors like [DB08873], [DB06290], or [DB05521]. When combined together, Grazoprevir and [DB11574] as the combination product Zepatier have been shown to achieve a SVR between 94% and 97% for genotype 1 and 97% and 100% for genotype 4 after 12 weeks of treatment. It can be used in patients with compensated cirrhosis, human immunodeficiency virus co-infection, or severe kidney disease. Grazoprevir anhydrous is a Hepatitis C Virus NS3/4A Protease Inhibitor. The mechanism of action of grazoprevir anhydrous is as a HCV NS3/4A Protease Inhibitor, and Breast Cancer Resistance Protein Inhibitor, and Cytochrome P450 3A Inhibitor. Drug Indication Grazoprevir is indicated in combination with [DB11574] (as the fixed dose combination product Zepatier) with or without [DB00811] for treatment of chronic HCV genotypes 1a, 1b, or 4 infection in adults. FDA Label Treatment of chronic hepatitis C Mechanism of Action Grazoprevir is a second generation NS3/4a protease inhibitor used to inhibit viral HCV replication. NS3/4a protease is an integral part of viral replication and mediates the cleavage the virally encoded polyprotein to mature proteins (NS3, NS4A, NS4B, NS5A and NS5B). Grazoprevir inhibits the NS3/4protease enzymes of HCV genotype 1a, 1B, and 4 with IC50 values of 7pM, 4pM, and 62pM, respectively. |
Solubility Data
| Solubility (In Vitro) |
DMSO : 50~100 mg/mL ( 65.20~130.39 mM ) Ethanol : 66.67~100 mg/mL(86.93 mM ) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (3.26 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (3.26 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 3: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (3.26 mM) Solubility in Formulation 4: 12.5 mg/mL (16.30 mM) in 100% PEG-300 (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3040 mL | 6.5198 mL | 13.0395 mL | |
| 5 mM | 0.2608 mL | 1.3040 mL | 2.6079 mL | |
| 10 mM | 0.1304 mL | 0.6520 mL | 1.3040 mL |