Physicochemical Properties
| Molecular Formula | C99H140N20O17 |
| Molecular Weight | 1882.29000 |
| Exact Mass | 1881.07 |
| Elemental Analysis | C, 63.17; H, 7.50; N, 14.88; O, 14.45 |
| CAS # | 11029-61-1 |
| Related CAS # | Gramicidin A TFA |
| PubChem CID | 16132269 |
| Appearance | White to off-white solid powder |
| LogP | 11.262 |
| Hydrogen Bond Donor Count | 21 |
| Hydrogen Bond Acceptor Count | 17 |
| Rotatable Bond Count | 52 |
| Heavy Atom Count | 136 |
| Complexity | 3980 |
| Defined Atom Stereocenter Count | 14 |
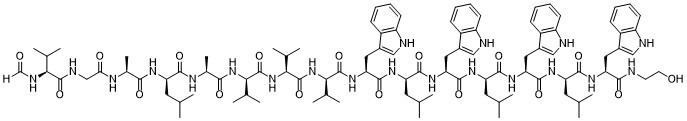
| SMILES | OCCNC([C@@H](NC(CNC([C@@H](NC(CNC([C@@H](NC(CNC([C@@H](NC([C@H](NC([C@@H](NC)C(C)C)=O)C(C)C)=O)CC1C2=CC=CC=C2NC=1)=O)=O)CC1C2=CC=CC=C2NC=1)=O)=O)CC1C2=CC=CC=C2NC=1)=O)=O)CC1C2=CC=CC=C2NC=1)=O |
| InChi Key | ZWCXYZRRTRDGQE-LUPIJMBPSA-N |
| InChi Code | InChI=1S/C99H140N20O17/c1-51(2)37-73(109-86(123)59(17)107-81(122)49-105-96(133)82(55(9)10)106-50-121)89(126)108-60(18)87(124)117-84(57(13)14)98(135)119-85(58(15)16)99(136)118-83(56(11)12)97(134)116-80(44-64-48-104-72-34-26-22-30-68(64)72)95(132)112-76(40-54(7)8)92(129)115-79(43-63-47-103-71-33-25-21-29-67(63)71)94(131)111-75(39-53(5)6)91(128)114-78(42-62-46-102-70-32-24-20-28-66(62)70)93(130)110-74(38-52(3)4)90(127)113-77(88(125)100-35-36-120)41-61-45-101-69-31-23-19-27-65(61)69/h19-34,45-48,50-60,73-80,82-85,101-104,120H,35-44,49H2,1-18H3,(H,100,125)(H,105,133)(H,106,121)(H,107,122)(H,108,126)(H,109,123)(H,110,130)(H,111,131)(H,112,132)(H,113,127)(H,114,128)(H,115,129)(H,116,134)(H,117,124)(H,118,136)(H,119,135)/t59-,60-,73+,74+,75+,76+,77-,78-,79-,80-,82-,83+,84+,85-/m0/s1 |
| Chemical Name | (2R)-2-[[(2S)-2-[[2-[[(2S)-2-formamido-3-methylbutanoyl]amino]acetyl]amino]propanoyl]amino]-N-[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-[[(2R)-1-[[(2S)-1-(2-hydroxyethylamino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]-4-methylpentanamide |
| Synonyms | Valinegramicidin A; Valyl gramicidin A; Gramicidin A; 11029-61-1; 1-L-Valinegramicidin A; 4419-81-2; Gramicidin A, 1-L-valine-; GNF-Pf-2578; 1-L-Valinegramicidin A; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | HIF-1α/hypoxia inducible factor 1 α |
| ln Vitro |
Strong broad-spectrum antibiotic activity is exhibited by Gramicidin A against Gram-positive strains, including strains that are resistant to multiple drugs[1].
One drawback of gramicidin A is its high hemolytic activity[1]. Similar to monensin (HY-N4302), gramicidin A (0.1 nM–10 μM, 72 h) decreases the viability of RCC cell lines[2]. Neither VHL nor HIF-1α expression significantly affects the cellular sensitivity of gramicidin A[2]. In RCC cells, gramicidin A (1 and 10 μM, 48 or 72 h) causes nonapoptotic cell death[2]. In RCC cells, gramicidin A (0–10 μM, 24 h) causes metabolic dysfunction and depletes cellular energy[2]. HIF-1α and HIF-2α protein expression, HIF transcriptional activity, and target gene expression are all decreased by gramicidin A (0–1 μM, 24-72 h)[3]. |
| ln Vivo |
RCC tumor xenografts are inhibited in growth by gramicidin A (0.11 mg/kg; intratumoral injection; twice weekly for 14 days)[2]. For 26 days, gramicidin A (0.22 mg/kg; intraperitoneal injection; three times weekly) prevents VHL-expressing RCC tumor xenografts from growing and angiogenizing[3]. |
| Cell Assay |
Cell Line: A498, 786-O, Caki-1, SN12C, ACHN, UMRC6, UMRC6+VHL, HEK293T+pcDNA3, HEK293T+HA-HIF-1α, HEK293T+HA-HIF-1α-mut Concentration: 0.1 nM-10 μM Incubation Time: 72 h Result: decreased the viability against A498, 786-O, Caki-1, SN12C, ACHN, UMRC6, UMRC6+VHL, HEK293T+pcDNA3, HEK293T+HA-HIF-1α, and HEK293T+HA-HIF-1α-mut cells with IC50s of 0.420, 0.430, 0.228, 0.104, 0.783, 0.253, 0.425, 0.057, 0.058, and 0.067 μM, respectively. |
| Animal Protocol |
Animal Model: Six to eight weeks old, female Nu/J mice without hair were given a subcutaneous injection of a 1.0 × 10^6 SN12C cell suspension in a 50% reduced growth factor Matrigel solution[2]. Dosage: 0.11 mg/kg body weight Administration: Intratumoral injection, twice weekly for 14 days Result: The average tumor mass was reduced by approximately 40% without significant toxicity. |
| Toxicity/Toxicokinetics |
16132269 mouse LD50 oral 1 gm/kg CRC Handbook of Antibiotic Compounds, Vols.1- , Berdy, J., Boca Raton, FL, CRC Press, 1980, 4(1)(240), 1980 16132269 mouse LD50 intraperitoneal 60 mg/kg CRC Handbook of Antibiotic Compounds, Vols.1- , Berdy, J., Boca Raton, FL, CRC Press, 1980, 4(1)(240), 1980 16132269 mouse LD50 intravenous 5 mg/kg CRC Handbook of Antibiotic Compounds, Vols.1- , Berdy, J., Boca Raton, FL, CRC Press, 1980, 4(1)(240), 1980 |
| References |
[1]. Discovery of gramicidin A analogues with altered activities by multidimensional screening of a one-bead-one-compound library. Nat Commun. 2020 Oct 1;11(1):4935. [2]. Gramicidin A induces metabolic dysfunction and energy depletion leading to cell death in renal cell carcinoma cells. Mol Cancer Ther. 2013 Nov;12(11):2296-307. [3]. Gramicidin A blocks tumor growth and angiogenesis through inhibition of hypoxia-inducible factor in renal cell carcinoma. Mol Cancer Ther. 2014 Apr;13(4):788-99. |
| Additional Infomation |
A group of peptide antibiotics from BACILLUS brevis. Gramicidin C or S is a cyclic, ten-amino acid polypeptide and gramicidins A, B, D are linear. Gramicidin is one of the two principal components of TYROTHRICIN. Gramicidin A (1) is a peptide antibiotic that disrupts the transmembrane ion concentration gradient by forming an ion channel in a lipid bilayer. Although long used clinically, it is limited to topical application because of its strong hemolytic activity and mammalian cytotoxicity, likely arising from the common ion transport mechanism. Here we report an integrated high-throughput strategy for discovering analogues of 1 with altered biological activity profiles. The 4096 analogue structures are designed to maintain the charge-neutral, hydrophobic, and channel forming properties of 1. Synthesis of the analogues, tandem mass spectrometry sequencing, and 3 microscale screenings enable us to identify 10 representative analogues. Re-synthesis and detailed functional evaluations find that all 10 analogues share a similar ion channel function, but have different cytotoxic, hemolytic, and antibacterial activities. Our large-scale structure-activity relationship studies reveal the feasibility of developing analogues of 1 that selectively induce toxicity toward target organisms. [1] Ionophores are lipid-soluble organic molecules that disrupt cellular transmembrane potential by rendering biologic membranes permeable to specific ions. They include mobile-carriers that complex with metal cations and channel-formers that insert into the membrane to form hydrophilic pores. Although mobile-carriers possess anticancer properties, investigations on channel-formers are limited. Here, we used the channel-forming ionophore gramicidin A to study its effects on the growth and survival of renal cell carcinoma (RCC) cells. RCC is a histologically heterogeneous malignancy that is highly resistant to conventional treatments. We found that gramicidin A reduced the in vitro viability of several RCC cell lines at submicromolar concentrations (all IC50 < 1.0 μmol/L). Gramicidin A exhibited similar toxicity in RCC cells regardless of histologic subtype or the expression of either the von Hippel-Lindau tumor suppressor gene or its downstream target, hypoxia-inducible factor-1α. Gramicidin A decreased cell viability equal to or greater than the mobile-carrier monensin depending on the cell line. Mechanistic examination revealed that gramicidin A blocks ATP generation by inhibiting oxidative phosphorylation and glycolysis, leading to cellular energy depletion and nonapoptotic cell death. Finally, gramicidin A effectively reduced the growth of RCC tumor xenografts in vivo. These results show a novel application of gramicidin A as a potential therapeutic agent for RCC therapy. [2] Ionophores are hydrophobic organic molecules that disrupt cellular transmembrane potential by permeabilizing membranes to specific ions. Gramicidin A is a channel-forming ionophore that forms a hydrophilic membrane pore that permits the rapid passage of monovalent cations. Previously, we found that gramicidin A induces cellular energy stress and cell death in renal cell carcinoma (RCC) cell lines. RCC is a therapy-resistant cancer that is characterized by constitutive activation of the transcription factor hypoxia-inducible factor (HIF). Here, we demonstrate that gramicidin A inhibits HIF in RCC cells. We found that gramicidin A destabilized HIF-1α and HIF-2α proteins in both normoxic and hypoxic conditions, which in turn diminished HIF transcriptional activity and the expression of various hypoxia-response genes. Mechanistic examination revealed that gramicidin A accelerates O(2)-dependent downregulation of HIF by upregulating the expression of the von Hippel-Lindau (VHL) tumor suppressor protein, which targets hydroxylated HIF for proteasomal degradation. Furthermore, gramicidin A reduced the growth of human RCC xenograft tumors without causing significant toxicity in mice. Gramicidin A-treated tumors also displayed physiologic and molecular features consistent with the inhibition of HIF-dependent angiogenesis. Taken together, these results demonstrate a new role for gramicidin A as a potent inhibitor of HIF that reduces tumor growth and angiogenesis in VHL-expressing RCC. [3] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.5313 mL | 2.6563 mL | 5.3127 mL | |
| 5 mM | 0.1063 mL | 0.5313 mL | 1.0625 mL | |
| 10 mM | 0.0531 mL | 0.2656 mL | 0.5313 mL |