Physicochemical Properties
| Molecular Formula | C19H39N5O7 |
| Molecular Weight | 449.54 |
| Exact Mass | 449.285 |
| CAS # | 26098-04-4 |
| PubChem CID | 72396 |
| Appearance | Typically exists as White to off-white solid at room temperature |
| Density | 1.36g/cm3 |
| Boiling Point | 675.2ºC at 760 mmHg |
| Melting Point | 102-108ºC |
| Flash Point | 362.1ºC |
| Vapour Pressure | 3.97E-21mmHg at 25°C |
| Index of Refraction | 1.603 |
| LogP | -5 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 31 |
| Complexity | 592 |
| Defined Atom Stereocenter Count | 12 |
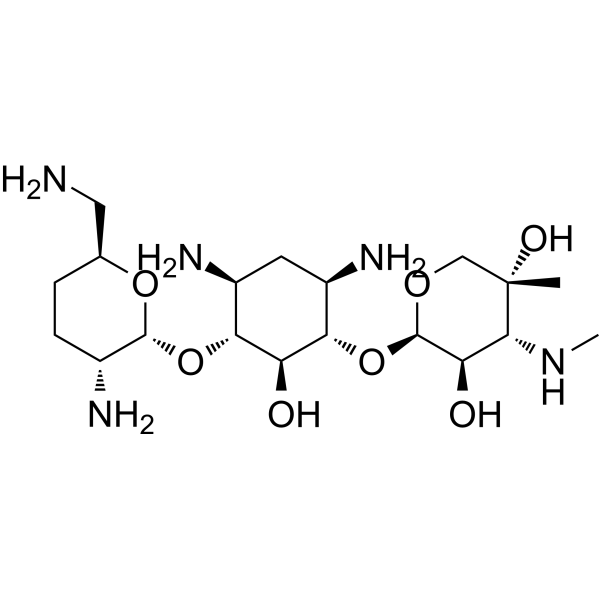
| SMILES | [NH3+]CC1CCC([NH3+])C(OC2C([NH3+])CC([NH3+])C(OC3OCC(C)(O)C([NH2+]C)C3O)C2O)O1 |
| InChi Key | VEGXETMJINRLTH-BOZYPMBZSA-N |
| InChi Code | InChI=1S/C19H39N5O7/c1-19(27)7-28-18(13(26)16(19)24-2)31-15-11(23)5-10(22)14(12(15)25)30-17-9(21)4-3-8(6-20)29-17/h8-18,24-27H,3-7,20-23H2,1-2H3/t8-,9+,10-,11+,12-,13+,14+,15-,16+,17+,18+,19-/m0/s1 |
| Chemical Name | (2R,3R,4R,5R)-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-(aminomethyl)oxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol |
| Synonyms | Gentamycin C1A; Gentamycin C12; Gentamicin Cla; AV4A72IATD; CHEBI:27784; (2R,3R,4R,5R)-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-(aminomethyl)oxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Antibiotic/antibacterial |
| ln Vitro | Gentamicin C1a has an IC50 value of 1 mg/mL and inhibits the growth of Escherichia coli, P. aeruginosa, and S. aureus[2]. OC-k3 cell viability is unaffected by gentamicin C1a (2 mM, 48 h) [2]. |
| ln Vivo | Gentamicin C1a (4 mg/kg, intravenous bolus dosage, single dose) had an average residence time of 84 minutes in beagle dogs and a CL value of 1.81 mL/min/kg[3]. |
| Enzyme Assay | Gentamicin C1a is the precursor of the semi-synthetic antibiotic etimicin and has the highest antibacterial activity in the clinically important gentamicin C mixture. To obtain a gentamicin C1a-overproducing strain, we inactivated gacD gene in Micromonospora purpurea. The gacD was presumed to encode a C6' methyltransferase by sequence analysis, and plays a role in the conversion of the gentamicin intermediate X2 to G418. So the inactivation of gacD blocks the metabolic pathways from X2 to G418 and leads to the accumulation of gentamicin C1a.The resulting recombination strain produced gentamicin C1a more than 10-fold compared to the wild type strain. Moreover, the wild-type strain produced 4 main production components, C1a, C2, C2a and C1, while the recombination strain produced only 2 components, C1a and C2b, making the purification of gentamicin C1a easier. The recombination strain was genetically stable and should be useful for the industrial production of gentamicin C1a[1]. |
| Cell Assay | Spread of antimicrobial resistance and shortage of novel antibiotics have led to an urgent need for new antibacterials. Although aminoglycoside antibiotics (AGs) are very potent anti-infectives, their use is largely restricted due to serious side-effects, mainly nephrotoxicity and ototoxicity. We evaluated the ototoxicity of various AGs selected from a larger set of AGs on the basis of their strong antibacterial activities against multidrug-resistant clinical isolates of the ESKAPE panel: gentamicin, gentamicin C1a, apramycin, paromomycin and neomycin. Following local round window application, dose-dependent effects of AGs on outer hair cell survival and compound action potentials showed gentamicin C1a and apramycin as the least toxic. Strikingly, although no changes were observed in compound action potential thresholds and outer hair cell survival following treatment with low concentrations of neomycin, gentamicin and paromomycin, the number of inner hair cell synaptic ribbons and the compound action potential amplitudes were reduced. This indication of hidden hearing loss was not observed with gentamicin C1a or apramycin at such concentrations. These findings identify the inner hair cells as the most vulnerable element to AG treatment, indicating that gentamicin C1a and apramycin are promising bases for the development of clinically useful antibiotics[2]. |
| Animal Protocol | The pharmacokinetics of gentamicin C(1), C(2), and C(1a) were studied in six beagles after administration of gentamicin at 4 mg/kg of body weight as a single intravenous bolus dose. Plasma concentrations of the gentamicin components were analyzed with a novel high-performance liquid chromatography method capable of identifying and quantifying each of the components. The pharmacokinetic analysis of the plasma concentration-versus-time data was performed using the noncompartmental approach. The results indicated significant differences in the pharmacokinetic characteristics between the gentamicin components C(1), C(1a), and C(2). The mean residence times of gentamicin C(1), C(1a), and C(2) were 81+/-13, 84+/-12, and 79+/-13 min (mean +/- standard deviation), respectively. The half-lives of the respective components were 64+/-12, 66+/-12 and 63+/-12 min. Clearance (CL) of gentamicin C(1), 4.62+/-0.71 ml min(-1) kg(-1), was significantly higher (P = 0.0156) than CL of gentamicin C(1a), 1.81+/-0.26 ml min(-1) kg(-1), and C(2), 1.82+/-0.25 ml min(-1) kg(-1). Similarly, the volume of distribution at steady state (V(ss)) of gentamicin C(1), 0.36+/-0.04 liter kg(-1), was significantly higher (P = 0.0156) than the V(ss) of gentamicin C(1a), 0.14+/-0.01 liter kg(-1), and C(2), 0.15+/-0.02 liter kg(-1). Tissue binding was considered the most likely cause for the difference. The difference may have clinical and toxicological significance.[3] |
| References |
[1]. Li D, et al. Construction of a gentamicin C1a-overproducing strain of Micromonospora purpurea by inactivation of the gacD gene. Microbiol Res. 2013 Jun 12;168(5):263-7. [2]. Ishikawa M, et al. Lower ototoxicity and absence of hidden hearing loss point to gentamicin C1a and apramycin as promising antibiotics for clinical use. Sci Rep. 2019 Feb 20;9(1):2410. [3]. Isoherranen N, et al. Pharmacokinetics of gentamicin C(1), C(1a), and C(2) in beagles after a single intravenous dose. Antimicrob Agents Chemother. 2000 Jun;44(6):1443-7. |
| Additional Infomation |
Gentamycin C1a is a gentamycin C. It is a conjugate base of a gentamycin C1a(5+). Gentamicin C1a has been reported in Cordyceps farinosa, Serratia plymuthica, and other organisms with data available. Gentamicin C1a is one of the major components of the gentamicin complex. Gentamicin C1a lacks methyl groups on the 2-amino-hexose ring and has a free amine at the 6' position. |
Solubility Data
| Solubility (In Vitro) | H2O : 250 mg/mL (556.12 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2245 mL | 11.1225 mL | 22.2450 mL | |
| 5 mM | 0.4449 mL | 2.2245 mL | 4.4490 mL | |
| 10 mM | 0.2224 mL | 1.1122 mL | 2.2245 mL |