GW7647 (GW-7647) is a novel, selective and potent agonist of human and murine PPARα4 with EC50s of 6 nM, 1.1 μM, and 6.2 μM for human PPARα, PPARγ and PPARδ, respectively. It was identified by assaying it for activity on human PPAR subtypes and employing solid-phase, parallel-array synthesis. Strong lipid-lowering activity in animal models of dyslipidemia was found for GW7647, a human PPARalpha agonist with about 200-fold selectivity over PPARgamma and PPARdelta. GW7647 is going to be a very useful chemical tool for researching the biology of PPARalpha in disease models in animals and human cells.
Physicochemical Properties
| Molecular Formula | C29H46N2O3S |
| Molecular Weight | 502.75214 |
| Exact Mass | 502.322 |
| Elemental Analysis | C, 69.28; H, 9.22; N, 5.57; O, 9.55; S, 6.38 |
| CAS # | 265129-71-3 |
| Related CAS # | 265129-71-3 |
| PubChem CID | 3392731 |
| Appearance | White to off-white solid powder |
| Density | 1.1±0.1 g/cm3 |
| Boiling Point | 693.9±55.0 °C at 760 mmHg |
| Flash Point | 373.5±31.5 °C |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.569 |
| LogP | 8.59 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 12 |
| Heavy Atom Count | 35 |
| Complexity | 636 |
| Defined Atom Stereocenter Count | 0 |
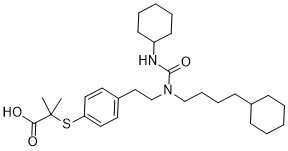
| SMILES | CC(C)(SC1=CC=C(CCN(C(NC2CCCCC2)=O)CCCCC3CCCCC3)C=C1)C(O)=O |
| InChi Key | PKNYXWMTHFMHKD-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C29H46N2O3S/c1-29(2,27(32)33)35-26-18-16-24(17-19-26)20-22-31(28(34)30-25-14-7-4-8-15-25)21-10-9-13-23-11-5-3-6-12-23/h16-19,23,25H,3-15,20-22H2,1-2H3,(H,30,34)(H,32,33) |
| Chemical Name | 2-[4-[2-[4-cyclohexylbutyl(cyclohexylcarbamoyl)amino]ethyl]phenyl]sulfanyl-2-methylpropanoic acid |
| Synonyms | GW 7647; GW-7647; GW7647 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PPARα (EC50 = 6 nM); Chk2 (IC50 = 697.4 nM); PPARγ (EC50 = 1.1 μM); PPARδ (EC50 = 6.2 μM) |
| ln Vitro |
GW7647 (1 μM) causes both in the presence and absence of IL-1β a significant increase in PDZK1 protein expression in Caco2BBE cells, reaching 129.7 ± 6.5% of vehicle treated control[1]. GW7647 also attenuates the PDZK1 expression decrease mediated by IL-1β. GW7647 (50 nM) increases the amounts of NO released by stimulating the phosphorylation of PI3K and then Akt (Ser473) in the stripped antral mucosa. In antral mucous cells, GW7647 (50 nM) amplifies the first stage of Ca2+-regulated exocytotic events triggered by ACh, but GW7647 by itself does not cause any exocytotic events. In antral mucous cells, GW7647 plus ACh increases the effects of wortmannin (50 nM) and AKT-inh (100 nM) on exocytotic events[2]. GW 7647 (100 nM) decreases the AQP9 protein abundance by 43%; however, at 10 and 1,000 nM, it has no discernible effec in WIF-B9 hepatocytes. HepG2 cells exposed to GW 7647 (100 nM) exhibit a 24% decrease in AQP9 protein abundance; however, L-FABP protein abundance in HepG2 hepatocytes is not significantly increased[3]. |
| ln Vivo | GW7647 (3 mg/kg per day) inhibits the decrease in left ventricular ejection fraction but does not stop the development of cardiac hypertrophy in vivo[4]. |
| Animal Protocol | New Zealand newborn Seven-day-old white rabbits weighing between 90 and 200 grams are anesthetized with 2% isofluorane inhaled, and they undergo an aorto-caval shunt to cause volume-overload cardiac hypertrophy. When using a color flow doppler on postoperative days 7 and 13, it is possible to see a physical shunt between the inferior vena cava and the abdominal aorta in both the axial and transverse planes, indicating the presence of a successful fistula. The enlarged inferior vena cava serves as additional confirmation of this. Following validation, animals in the shunt group are randomized to receive either GW7647 (3 mg/kg per day; EC50=6 nM for PPARα) or the vehicle (dimethyl sulfoxide, the solvent of GW7647) twice daily for 14 days via intraperitoneal injection. Animals that have surgery to create a shunt are not allowed to continue in the study if the shunt closes or does not exhibit. Transthoracic echocardiography is used to measure the left ventricular ejection fraction (%) and other cardiac parameters on postoperative days 7 and 13. All animals are put to sleep with Na+ pentobarbital at age 21 (14 days after surgery), and their hearts are removed for isolated biventricular working heart perfusions. |
| References |
[1]. IL-1β-Induced Downregulation of the Multifunctional PDZ Adaptor PDZK1 Is Attenuated by ERK Inhibition, RXRα, or PPARα Stimulation in Enterocytes. Front Physiol. 2017 Feb 7;8:61. [2]. PPARα induced NOS1 phosphorylation via PI3K/Akt in guinea pig antral mucous cells: NO-enhancement in Ca(2+)-regulated exocytosis. Biomed Res. 2016;37(3):167-78. [3]. Hepatic AQP9 expression in male rats is reduced in response to PPARα agonist treatment. Am J Physiol Gastrointest Liver Physiol. 2015 Feb 1;308(3):G198-205. [4]. Activating PPARα prevents post-ischemic contractile dysfunction in hypertrophied neonatal hearts. Circ Res. 2015 Jun 19;117(1):41-51. [5]. Identification of a subtype selective human PPARalpha agonist through parallel-array synthesis. Bioorg Med Chem Lett. 2001 May 7;11(9):1225-7. |
| Additional Infomation | GW 7647 is a monocarboxylic acid that is 2-(phenylsulfanyl)isobutyric acid in which the phenyl group is substituted at the para- position by a 3-aza-7-cyclohexylhept-1-yl group in which the nitrogen is acylated by a (cyclohexylamino)carbonyl group. It has a role as a PPARalpha agonist. It is a member of ureas, an aryl sulfide and a monocarboxylic acid. |
Solubility Data
| Solubility (In Vitro) |
DMSO: ~100 mg/mL (~198.9 mM) Ethanol: ~25 mg/mL (~49.7 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.97 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.97 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9891 mL | 9.9453 mL | 19.8906 mL | |
| 5 mM | 0.3978 mL | 1.9891 mL | 3.9781 mL | |
| 10 mM | 0.1989 mL | 0.9945 mL | 1.9891 mL |