Physicochemical Properties
| Molecular Formula | C20H17F2N5O2 |
| Molecular Weight | 397.378090620041 |
| Exact Mass | 397.135 |
| Elemental Analysis | C, 60.45; H, 4.31; F, 9.56; N, 17.62; O, 8.05 |
| CAS # | 1622849-43-7 |
| Related CAS # | 1622849-43-7 |
| PubChem CID | 118557502 |
| Appearance | White to off-white solid powder |
| LogP | 3.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 29 |
| Complexity | 603 |
| Defined Atom Stereocenter Count | 1 |
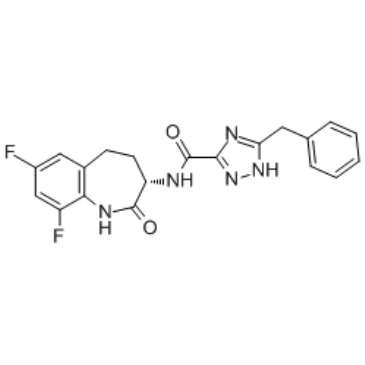
| SMILES | C1CC2=C(C(=CC(=C2)F)F)NC(=O)[C@H]1NC(=O)C3=NNC(=N3)CC4=CC=CC=C4 |
| InChi Key | ATQAGKAMBISZQM-HNNXBMFYSA-N |
| InChi Code | InChI=1S/C20H17F2N5O2/c21-13-9-12-6-7-15(19(28)25-17(12)14(22)10-13)23-20(29)18-24-16(26-27-18)8-11-4-2-1-3-5-11/h1-5,9-10,15H,6-8H2,(H,23,29)(H,25,28)(H,24,26,27)/t15-/m0/s1 |
| Chemical Name | 5-benzyl-N-[(3S)-7,9-difluoro-2-oxo-1,3,4,5-tetrahydro-1-benzazepin-3-yl]-1H-1,2,4-triazole-3-carboxamide |
| Synonyms | GSK3145095; GSK-3145095; GSK 3145095; 1622849-43-7; UNII-B4D3WPS7JY; B4D3WPS7JY; GSK-3145095; 5-benzyl-N-[(3S)-7,9-difluoro-2-oxo-1,3,4,5-tetrahydro-1-benzazepin-3-yl]-1H-1,2,4-triazole-3-carboxamide; CHEMBL4452233; (S)-5-benzyl-N-(7,9-difluoro-2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-3-yl)-4H-1,2,4-triazole-3-carboxamide; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | RIP1 kinase (IC50 = 6.3 nM) |
| ln Vitro | GSK3145095 has excellent activity in blocking RIP1 kinasedependent cellular responses and potently binds to RIP1 with exquisite kinase specificity. The inhibitor is also capable of encouraging a tumor suppressive T cell phenotype in pancreatic adenocarcinoma organ cultures, highlighting its potential as a novel cancer therapy. [1] |
| ln Vivo |
GSK3145095 also increased the survival rate (50%), but less than RI-962. Treatment with RI-962 or GSK3145095 remarkably reduced the TNFα-induced temperature loss (Fig. 8b) and the concentrations of proinflammatory cytokines (IL-1β and IL-6) in mice [2]. DSS treatment led to a rapid loss in mouse body weight from day 5 to day 11, and treatment with RI-962 or GSK3145095 strongly ameliorated this loss of body weight. Further, treatment with RI-962 or GSK3145095 markedly reduced the DSS-induced shortening of colon length (Fig. 9b, c). Histopathological analysis showed that RI-962 substantially decreased tissue damage in the colons of DSS-treated mice (Fig. 9d). In DSS-induced colitis, numerous S100a9-positive cells (a marker of inflammation) infiltrated into the mucosa and epithelial layer of the damaged colon (Fig. 9e), while no infiltration by S100a9-positive cells was observed in the colons of mice treated with RI-962 (Fig. 9e). More importantly, treatment with RI-962 or GSK3145095 dramatically increased the survival rate of DSS-treated mice (Fig. 9f; 40 mg/kg RI-962 or GSK3145095 survival rate, 100% vs vehicle: 16.7%) [2]. |
| Enzyme Assay |
ADP-Glo Activity Assay. [1] The catalytic activity of RIP1 was quantified utilizing the Promega ADP-Glo kinase kit as previously described (Harris et al, 2016) using either a four-parameter curve fit or a tight binding curve fit for compounds whose potency was less than the detection limit of the assay (~ half the enzyme concentration). Data are presented as the mean IC50 from at least n=2 determinations[1]. Compound 6 (GSK3145095) kinase selectivity and species selectivity profiles [1] Percent Enzyme Inhibition against Reaction Biology Corporation (RBC) kinase Panel [1] Compound 6 was tested at 10 M in duplicate against 359 kinases in the Reaction Biology Corporation (RBC) kinase panel.Control compound was tested in 10-dose IC50 mode with 3-fold serial dilution starting at 20 μM . Reactions were carried out at 10 μM ATP. Full protocol details are available at http://www.reactionbiology.com. Data is reported as % enzyme activity (relative to DMSO controls) in Table S1. Activity <50% (average of n =2) was observed for ABL1, ABL2/ARG, BLK, c-Src, DDR2, EPHA5, EPHB2, FGR, FRK/PTK5, FYN, LYN, LYN B, PEAK1 and YES/YES1. However full curve analysis for these 14 kinases against compound 6 at a top concentration of 30 M (see Table S2) found no inhibition indicating the initial single concentration findings were false positives. The kinase panel did not include RIP1. |
| Cell Assay | GSK3145095 is prepared in assay buffer, serially diluted 1:1.5 in a 22 point titration (high final concentration 3 μM), and added to a 384 white low volume Greiner plate. In assay buffer, 3.5 μL of each inhibitor concentration and 3.5 L of final 25 nM enzyme concentration are added to the plate. Following these additions, 3.5 μL of ATP (15.6 μM to 875 μM final) in assay buffer is added to the plate to start the reaction. At room temperature, the reaction proceeds for five hours. |
| Animal Protocol |
The DSS-induced IBD experiment[2] DSS (3% w/v) was administered in drinking water ad libitum for 7 d (from day 0 to day 7). DSS solution was replaced three times on day 2, day 4, and day 6. C57BL/6 female mice were injected intraperitoneally with vehicle, RI-962 (40 mg/kg), or GSK3145095 (40 mg/kg) for 10 d (from day 0 to day 9). Three mice in each group were killed at random on day 7, and distal colon tissues were collected for analysis. The mice weight and survival rate were recorded daily. |
| References |
[1]. ACS Med Chem Lett. 2019 May 9;10(6):857-862. [2]. Generative deep learning enables the discovery of a potent and selective RIPK1 inhibitor. Nat Commun . 2022 Nov 12;13(1):6891. |
| Additional Infomation | RIPK1 Inhibitor GSK3145095 is an orally available, small-molecule inhibitor of receptor-interacting serine/threonine-protein kinase 1 (RIPK1; receptor-interacting protein 1; RIP1) with potential antineoplastic and immunomodulatory activities. Upon administration, GSK3145095 disrupts RIPK1-mediated signaling, which may reduce C-X-C motif chemokine ligand 1 (CXCL1)-driven recruitment and migration of immunosuppressive myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment (TME). This allows effector cells, such as natural killer (NK) cells and cytotoxic T-lymphocytes (CTLs), to kill and eliminate cancer cells. RIPK1, a serine-threonine kinase that normally plays a key role in inflammation and cell death in response to tissue damage and pathogen recognition, is overexpressed in certain cancer types and may be associated with oncogenesis and promotion of the immunosuppressive nature of the TME. |
Solubility Data
| Solubility (In Vitro) |
DMSO: 250~79 mg/mL (~198.8 mM) Ethanol: ~6 mg/mL (~15.1 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (5.23 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (5.23 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (5.23 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5165 mL | 12.5824 mL | 25.1648 mL | |
| 5 mM | 0.5033 mL | 2.5165 mL | 5.0330 mL | |
| 10 mM | 0.2516 mL | 1.2582 mL | 2.5165 mL |