GSK2982772 (GSK-2982772; GSK772) is a potent, selective, oral and ATP competitive inhibitor of RIP1 (receptor Interacting Protein 1) with the potential for the treatment of inflammatory diseases. With an IC50 of 16 nM, it blocks RIP1 kinase. It was found in a DNA-encoded library and is presently undergoing a phase 2 clinical trial for ulcerative colitis, psoriatic arthritis, and rheumatoid arthritis. RIP1 controls inflammation and necroptosis, and it may be a major factor in a number of human pathologies, including immune-mediated inflammatory diseases. GSK2982772 shows more than 1,000-fold selectivity for ERK5 over a panel of over 339 kinases at 10 μM. GSK2982772 can inhibit the production of cytokines (IL-1β and IL-6) spontaneously from ulcerative colitis explant tissue in stimulated cellular systems in a concentration-dependent manner over the course of an overnight incubation. In human embryonic kidney (HEK-293) cells, GSK2982772 exhibits a weakly activating effect on the human Pregnane X receptor (hPXR) with an EC50 of 13 μM.It also exhibits a weakly concentration-dependent inhibition of hERG with an estimated IC50 of 195 μM.
Physicochemical Properties
| Molecular Formula | C20H19N5O3 | |
| Molecular Weight | 377.40 | |
| Exact Mass | 377.148 | |
| Elemental Analysis | C, 63.65; H, 5.07; N, 18.56; O, 12.72 | |
| CAS # | 1622848-92-3 | |
| Related CAS # | 1622848-92-3;1987858-31-0 (hydrate); | |
| PubChem CID | 77108121 | |
| Appearance | White to yellow solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Index of Refraction | 1.676 | |
| LogP | 2.08 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 28 | |
| Complexity | 568 | |
| Defined Atom Stereocenter Count | 1 | |
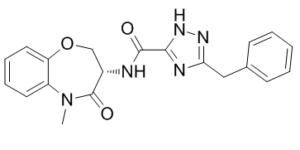
| SMILES | O1C2=C([H])C([H])=C([H])C([H])=C2N(C([H])([H])[H])C([C@]([H])(C1([H])[H])N([H])C(C1=NN([H])C(C([H])([H])C2C([H])=C([H])C([H])=C([H])C=2[H])=N1)=O)=O |
|
| InChi Key | LYPAFUINURXJSG-AWEZNQCLSA-N | |
| InChi Code | InChI=1S/C20H19N5O3/c1-25-15-9-5-6-10-16(15)28-12-14(20(25)27)21-19(26)18-22-17(23-24-18)11-13-7-3-2-4-8-13/h2-10,14H,11-12H2,1H3,(H,21,26)(H,22,23,24)/t14-/m0/s1 | |
| Chemical Name | 5-benzyl-N-[(3S)-5-methyl-4-oxo-2,3-dihydro-1,5-benzoxazepin-3-yl]-1H-1,2,4-triazole-3-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | human RIP1 FP (IC50 = 16 nM); monkey RIP1 FP (IC50 = 20 nM); rat RIP1 FP (IC50 = 2 μM); mouse RIP1 FP (IC50 = 2.5 μM) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | GSK2982772 is a potent, selective, oral and ATP competitive inhibitor of RIP1 (receptor Interacting Protein 1) with an IC50 of 16 nM. | ||
| Cell Assay | GSK2982772 shows more than 1,000-fold selectivity for ERK5 over a panel of over 339 kinases at 10 μM. In stimulated cellular systems,GSK2982772 is also able to reduce spontaneous production of cytokines (IL-1β and IL-6) in a concentration-dependent fashion from ulcerative colitis explant tissue in overnight incubations. GSK2982772 produces a weak concentration dependent inhibition of hERG in human embryonic kidney (HEK-293) cells, with an estimated IC50 of 195 μM, and also shows a weak activation of the human Pregnane X receptor (hPXR) with an EC50 of 13 μM. | ||
| Animal Protocol |
|
||
| References |
[1]. Discovery of a First-in-Class Receptor Interacting Protein 1 (RIP1) Kinase Specific Clinical Candidate (GSK2982772) for the Treatment of Inflammatory Diseases. J Med Chem. 2017 Feb 23;60(4):1247-1261. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.62 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 2: ≥ 2.08 mg/mL (5.51 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (5.51 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6497 mL | 13.2485 mL | 26.4971 mL | |
| 5 mM | 0.5299 mL | 2.6497 mL | 5.2994 mL | |
| 10 mM | 0.2650 mL | 1.3249 mL | 2.6497 mL |