GSKJ1 (GSK-J1; GSK-J 1; GSK-J-1) is a novel, highly selective and potent inhibitor of histone demethylase (H3K27me3/me2-demethylases JMJD3/KDM6B and UTX/KDM6A) with potential antineoplastic activity. It that has significant activity (IC50 60 nM for human JmjD3) in vitro and in cell assays using an ester prodrug derivative (GSK-J4: 1 µM < IC50 < 10 µM; e.g. 9 µM in primary human macrophages). The methyl groups from tri- and dimethylated lysine 4 of histone H3 are removed by the KDM5/JARID1 family of Fe(II)- and α-ketoglutarate-dependent demethylases. KDM5A (JARID1A/RBP2) and KDM5B (JARID1B/PLU1) may play a role as oncogenic drivers, according to mounting evidence from primary tumors and model systems. As an ester derivative (GSK-J5) in cells and a control for target effects in vitro, the pyridine regioisomer GSK-J2 exhibits significantly less on-target activity (IC50 > 100 µM for human JmjD3). Recent data against H3K4me3/2/1 demethylases indicates that GSK-J1 also exhibits some activity (IC50 950 nM for Jarid1b, IC50 1.76 uM for Jarid1c).
Physicochemical Properties
| Molecular Formula | C22H23N5O2 | |
| Molecular Weight | 389.45 | |
| Exact Mass | 389.185 | |
| Elemental Analysis | C, 67.85; H, 5.95; N, 17.98; O, 8.22 | |
| CAS # | 1373422-53-7 | |
| Related CAS # |
|
|
| PubChem CID | 56963315 | |
| Appearance | White to yellow solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 608.9±55.0 °C at 760 mmHg | |
| Flash Point | 322.0±31.5 °C | |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C | |
| Index of Refraction | 1.653 | |
| LogP | 2.75 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 7 | |
| Rotatable Bond Count | 6 | |
| Heavy Atom Count | 29 | |
| Complexity | 517 | |
| Defined Atom Stereocenter Count | 0 | |
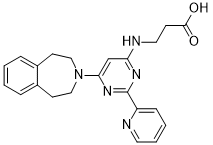
| SMILES | O([H])C(C([H])([H])C([H])([H])N([H])C1=C([H])C(=NC(C2=C([H])C([H])=C([H])C([H])=N2)=N1)N1C([H])([H])C([H])([H])C2=C([H])C([H])=C([H])C([H])=C2C([H])([H])C1([H])[H])=O |
|
| InChi Key | AVZCPICCWKMZDT-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C22H23N5O2/c28-21(29)8-12-24-19-15-20(26-22(25-19)18-7-3-4-11-23-18)27-13-9-16-5-1-2-6-17(16)10-14-27/h1-7,11,15H,8-10,12-14H2,(H,28,29)(H,24,25,26) | |
| Chemical Name | 3-[[2-pyridin-2-yl-6-(1,2,4,5-tetrahydro-3-benzazepin-3-yl)pyrimidin-4-yl]amino]propanoic acid | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | JMJD3 ( IC50 = 60 nM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | Purified JmjD3 (1 μM) and UTX (3 μM) are incubated with a 10 μM peptide [Biotin-KAPRKQLATKAARK(me3)SAPATGG]. in 50 mM HEPES pH 7.5, 150 mM KCl, 50μM (NH4)2SO4·FeSO4·H2O, 1 mM 2-oxoglutarate, and 2 mM ascorbate (JmjD3, 3 minutes at 25°C; UTX, 20 minutes at 25°C) had different inhibitor concentrations (0, 0.005, 0.01, 0.02, 0.05, 0.1 μM). To halt the reaction, add 10 mM EDTA. Utilizing a zip tip to desalt the reaction, the reaction is then spotted using an α-cyano-4-hydroxycinnamic acid MALDI matrix on a MALDI plate. Via the MALDI-TOF R system, samples are analyzed. | ||
| Animal Protocol |
|
||
| References |
[1]. Nature . 2012 Aug 16;488(7411):404-8. [2]. J Cell Biochem . 2015 Nov;116(11):2628-36. [3]. J Biol Chem . 2022 Jun;298(6):102017. |
||
| Additional Infomation | 3-[[2-(2-pyridinyl)-6-(1,2,4,5-tetrahydro-3-benzazepin-3-yl)-4-pyrimidinyl]amino]propanoic acid is an organonitrogen heterocyclic compound. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.42 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.42 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5677 mL | 12.8386 mL | 25.6772 mL | |
| 5 mM | 0.5135 mL | 2.5677 mL | 5.1354 mL | |
| 10 mM | 0.2568 mL | 1.2839 mL | 2.5677 mL |