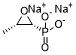Physicochemical Properties
| Molecular Formula | C3H5NA2O4P |
| Molecular Weight | 182.02 |
| Exact Mass | 181.972 |
| Elemental Analysis | C, 19.80; H, 2.77; Na, 25.26; O, 35.16; P, 17.02 |
| CAS # | 26016-99-9 |
| Related CAS # | Fosfomycin calcium;26016-98-8;Fosfomycin tromethamine;78964-85-9;Fosfomycin;23155-02-4 |
| PubChem CID | 73491 |
| Appearance | White to off-white solid powder |
| Boiling Point | 342.7ºC at 760 mmHg |
| Melting Point | >300°C |
| Vapour Pressure | 1.34E-05mmHg at 25°C |
| Source | Streptomyces spp. |
| LogP | 0.785 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Heavy Atom Count | 10 |
| Complexity | 127 |
| Defined Atom Stereocenter Count | 2 |
| SMILES | P([C@]1([H])[C@@]([H])(C([H])([H])[H])O1)(=O)([O-])[O-].[Na+].[Na+] |
| InChi Key | QZIQJIKUVJMTDG-JSTPYPERSA-L |
| InChi Code | InChI=1S/C3H7O4P.2Na/c1-2-3(7-2)8(4,5)6;;/h2-3H,1H3,(H2,4,5,6);;/q;2*+1/p-2/t2-,3+;;/m0../s1 |
| Chemical Name | Phosphonic acid, (3-methyloxiranyl)-, disodium salt, (2R-cis)- (9CI) |
| Synonyms | Disodium fosfomycin; Disodium phosphonomycin; Forocyle S; Fosfomycin sodium; MK 955; MK955; MK-955; Phosphonomycin disodium salt |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Cell wall synthesis |
| ln Vitro | Fosfomycin sodium is an antibacterial agent for epoxy. In contrast to other antibacterial drugs, its mechanism of action involves impeding the first stage of cell wall formation [1]. With a 90% inhibition rate, fosfomycin sodium exhibits bactericidal efficacy against a range of Gram-positive and Gram-negative pathogenic bacteria, including β-bacteria that produce carbapenemase and extended-spectrum lactamases[1]. Because of its wide tissue penetration, fosfomycin sodium is useful in the research of infections pertaining to the lungs, soft tissue, bone, central nervous system, and abscesses [2]. |
| ln Vivo | The administration of fosfomycin sodium (80 mg/kg; IV-IV or IV-PO) does not alter its protective effect against double bekacin nephrotoxicity [3]. Folsfomycin sodium pharmacokinetics in rats [4] Dibekacin dosage (mg) Vdss (l/kg) β (min-1) T1/2 (min) Urinary recovery rate (%) 30 0.261 0.0244 28.4 85.[hr] Protection by fosfomycin of the nephrotoxicity of dibekacin was studied using Fischer 344 rats and urinary parameters such as volume, osmolality, protein, N-acetyl-beta-D-glucosaminidase, leucine aminopeptidase, lactate dehydrogenase and nucleated cells were determined as markers of nephrotoxicity. The duration of treatment was 11 d. Fosfomycin reduced polyuria, proteinuria, enzymuria and cyturia induced by dibekacin best by the concomitant administration, followed by pre-treatment, but not by post-treatment. Protection was effective in the dose ratio of dibekacin: fosfomycin = 1:2 - 1:32, regardless of administration routes. As judged from urinalysis, protection by fosfomycin (320 mg/kg) was almost complete for the experimental nephrotoxicity induced by 10 mg/kg of dibekacin, and still significant for that by 40 mg/kg. This was supported by the histo-pathological and ultrastructural improvement of proximal tubules and by suppressed blood urea nitrogen and creatinine values. Protective activity of fosfomycin was more potent than that of cephalothin, when compared on the weight basis [3]. |
| Enzyme Assay | Fosfomycin is a bactericidal antibiotic agent. It inhibits an enzyme-catalyzed reaction in the first step of the synthesis of the bacterial cell wall. Fosfomycin interferes with the first cytoplasmic step of bacterial cell wall biosynthesis, the formation of the peptidoglycan precursor UDP N-acetylmuramic acid (UDP-MurNAc). Specifically, the enzyme UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) is involved in peptidoglycan biosynthesis by catalyzing the transfer of the enolpyruvyl moiety of phosphoenolpyruvate (PEP) to the 3′-hydroxyl group of UDP-N-acetylglucosamine (UNAG). Fosfomycin covalently binds to the thiol group of a cysteine (position 115 in Escherichia coli numbering; target Cys115) in the active site of MurA and consequently inactivates it. This inhibitory action takes place at an earlier step than the action of β-lactams or glycopeptides [1]. |
| Cell Assay | Fosfomycin exerts immunomodulatory effects by altering lymphocyte, monocyte and neutrophil function. It affects the acute inflammatory cytokine response in vitro and in vivo. It suppresses production of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-1α and increases production of IL-10, while contradictory data have been published regarding IL-6. On the other hand, concentrations of TNF-α, IL-1β, and IL-6 expressed as protein and mRNA were almost identical with and without fosfomycin in healthy volunteers. Fosfomycin suppresses IL-2 production from T cells, the production of leukotriene B4 (LTB4) from neutrophils, and the expression of IL-8 mRNA by LTB4 from monocytes. Fosfomycin also exhibits an immunomodulatory effect on B-cell activation. Fosfomycin enhances neutrophil phagocytic killing of invading pathogens, even in patients on chronic hemodialysis and renal transplantation). Fosfomycin resulted in enhanced bactericidal ability of neutrophils compared to other antimicrobials. The clinical relevance of the aforementioned actions remains to be elucidated [1]. |
| Animal Protocol |
Animal/Disease Models: Fischer 344 rats [3 ] Doses: 320 mg/kg Route of Administration: intramuscularinjection, 5 courses of treatment: 1 hour, 0.5 hrs (hrs (hours)) earlier than debekacin, at the same time, 0.5 hrs (hrs (hours)) later, 1 hour late; 11-day Experimental Results: Following previous treatment, polyuria, proteinuria, enzymes, and cytosine diminished due to dibekacin (40 mg/kg). Animal/Disease Models: Acute renal failure dehydrated Wistar rats (8 weeks old) [4] Doses: 120 mg/kg Route of Administration: intravenous (iv) (iv)injection; 200mg/kg. The first Experimental Results:the elimination rate of rats basically returned to normal, and the nephrotoxicity parameters improved. Protects proximal tubular lysosomes from the effects of aminoglycosides by inhibiting myelopoiesis and protecting the integrity of lysosomal membranes in rats treated with bibekacin. We studied the mechanism on protective effect of fosfomycin against experimental nephrotoxicity induced by dibekacin. In order to simplify an experimental model, the dehydrated Wistar rats were used, because a single injection of dibekacin at 30 mg/kg induced acute renal failure in the dehydrated rats, characterized by alteration of urinalytic parameters and BUN values, and retarded elimination of dibekacin from blood. When the rats were administered simultaneously with fosfomycin at 120 mg/kg, the rate of elimination was restored almost to normal, accompanied with improvement of the nephrotoxic parameters. However, markedly accelerated elimination over normal one was not observed, indicating that the improved elimination was not the reason of protection but a result of normal kidney function. On the other hand, fosfomycin protected the proximal tubular lysosomes from the injury of aminoglycoside, as evidenced a) in vivo by suppression of myeloid body formation and protection of lysosomal membrane integrity of the rats treated with dibekacin, and b) in vitro by dose-dependent protection of the lysosomal membrane integrity of the kidney cells. A study of structure-protective activity relation revealed that phosphonate anion possessing an epoxy function was important for protection, and that the mechanism of protection differed from the antibacterial mechanism [4]. |
| References |
[1]. Fosfomycin. Clin Microbiol Rev. 2016 Apr. 29(2):321-47. [2]. Fosfomycin: Pharmacological, Clinical and Future Perspectives. Antibiotics (Basel). 2017 Oct 31. 6(4):24. [3]. Protective effect of fosfomycin on the experimental nephrotoxicity induced by dibekacin. J Pharmacobiodyn. 1982 Sep. 5(9):659-69. [4]. Mode of protective action of fosfomycin against dibekacin-induced nephrotoxicity in the dehydrated rats. J Pharmacobiodyn. 1982 Dec. 5(12):941-50. |
Solubility Data
| Solubility (In Vitro) | Water : 36~125 mg/mL(686.74 mM ) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 100 mg/mL (549.39 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.4939 mL | 27.4695 mL | 54.9390 mL | |
| 5 mM | 1.0988 mL | 5.4939 mL | 10.9878 mL | |
| 10 mM | 0.5494 mL | 2.7470 mL | 5.4939 mL |