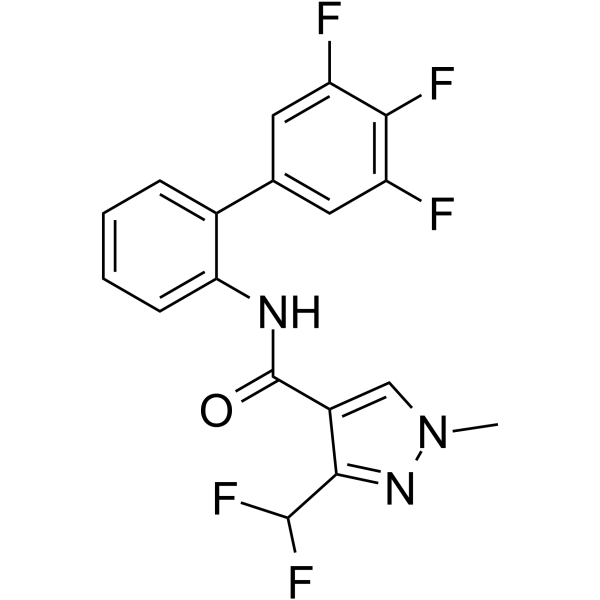
Fluxapyroxad is a novel, broad-spectrumand potent fungicidal agent acting as a succinate dehydrogenase inhibitor (SDHI). Working by interfering with a number of key fungal life functions, including spore germination, germ tube growth, appresoria formation and mycelium growth.
Physicochemical Properties
| Molecular Formula | C18H12F5N3O |
| Molecular Weight | 381.299401283264 |
| Exact Mass | 381.09 |
| Elemental Analysis | C, 56.70; H, 3.17; F, 24.91; N, 11.02; O, 4.20 |
| CAS # | 907204-31-3 |
| PubChem CID | 16095400 |
| Appearance | White to off-white solid powder |
| Density | 1.42g/cm3 |
| Boiling Point | 428.4ºC at 760 mmHg |
| Melting Point | 156.8 ℃ |
| Flash Point | 212.9ºC |
| Index of Refraction | 1.57 |
| LogP | 4.767 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 27 |
| Complexity | 513 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=C(C1C(C(F)F)=NN(C)C=1)NC1C(C2C=C(F)C(F)=C(F)C=2)=CC=CC=1 |
| InChi Key | SXSGXWCSHSVPGB-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C18H12F5N3O/c1-26-8-11(16(25-26)17(22)23)18(27)24-14-5-3-2-4-10(14)9-6-12(19)15(21)13(20)7-9/h2-8,17H,1H3,(H,24,27) |
| Chemical Name | 3-(Difluoromethyl)-1-methyl-N-[2-(3,4,5-trifluorophenyl)phenyl]pyrazole-4-carboxamide |
| Synonyms | Fluxapyroxad |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Succinate dehydrogenase |
| ln Vitro | Qualitative analysis and quantification of pesticide residues in foodstuff are essential to our health in daily life, especially regarding their metabolites, which may be more toxic and persistent. Thus, a valid analytical measure for detection of fluxapyroxad and its three metabolites (M700F002 (C-2), M700F008 (C-8), M700F048 (C-48)) in vegetables (cucumber, tomato, and pepper), fruits (grape, apple), and cereals (wheat, rice) was developed by UPLC-MS/MS with negative ion mode. The target compounds were extracted by acetonitrile contain 0.2% formic acid (v/v), and the extractions were cleaned up by octadecylsilane sorbents. The limits of quantitation and quantification were less than 0.14 μg kg-1 and 0.47 μg kg-1 in seven matrices. Furthermore, recoveries at levels of 0.01, 0.05, and 0.1 mg kg-1 ranged from 74.9% to 110.5% with relative standard deviations ≤15.5% (n = 5). The method is validated to be effective and robust for the routine supervising of fluxapyroxad and its metabolites [1]. |
| ln Vivo | Fluxapyroxad (FX), a succinate dehydrogenase inhibitor fungicide, has been detected in global marine and aquatic organisms. However, as a new pollutant, its biotoxicity and ecological risks to marine aquatic organisms are unclear. The accumulation and elimination processes and toxic effects of FX on Larimichthys crocea (L. crocea) at environmental concentrations were assessed. FX (1.0 μg/L) was rapidly enriched and persisted prolonged in L. crocea muscle and FX is highly toxic to juvenile L. crocea with the 96 h LC50 of 245.0 μg/L. Furthermore, the toxic effects of FX on juvenile L. crocea and adults L. crocea were compared and analyzed. In contrast to those of adult L. crocea, juvenile L. crocea showed a stronger oxidative stress response and rescued liver damage in terms of antioxidant enzyme activity, energy supply, and liver damage to FX. Transcriptomic analysis also showed that drug metabolism was activated. In the adult L. crocea, the disturbance of the energy metabolism, oxidative respiration, TCA cycle, and lipid metabolism genes were firstly found. The results revealed the accumulation and elimination pattern and ecotoxicological hazards of FX to L. crocea, which provided important theoretical basis for the study of environmental risks caused by new pollutants to marine organisms [2]. |
| References |
[1]. Effective Monitoring of Fluxapyroxad and Its Three Biologically Active Metabolites in Vegetables, Fruits, and Cereals by Optimized QuEChERS Treatment Based on UPLC-MS/MS. J Agric Food Chem. 2016 Nov 23;64(46):8935-8943. [2]. Accumulation and elimination properties and comparative toxicity of fluxapyroxad in juvenile and adult large yellow croaker (Larimichthys crocea). Sci Total Environ. 2024 Feb 20:912:168979. |
| Additional Infomation | Fluxapyroxad is an aromatic amide obtained by formal condensation of the carboxy group of 3-(difluoromethyl)-1-methylpyrazole-4-carboxylic acid with the amino group of 3',4',5'-trifluorobiphenyl-2-amine. Used to control a number of cereal fungal pathogens including those belonging to the Ascomycetes, Basidiomycetes and Zygomycetes families. It has a role as an EC 1.3.5.1 [succinate dehydrogenase (quinone)] inhibitor and an antifungal agrochemical. It is an aromatic amide, a member of biphenyls, a member of pyrazoles, a trifluorobenzene and an anilide fungicide. |
Solubility Data
| Solubility (In Vitro) | DMSO: ≥ 100 mg/mL (~262.26 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6226 mL | 13.1130 mL | 26.2261 mL | |
| 5 mM | 0.5245 mL | 2.6226 mL | 5.2452 mL | |
| 10 mM | 0.2623 mL | 1.3113 mL | 2.6226 mL |