Physicochemical Properties
| Molecular Formula | C17H12CLN5O2S |
| Molecular Weight | 385.827480316162 |
| Exact Mass | 385.04 |
| CAS # | 2050524-24-6 |
| PubChem CID | 141461656 |
| Appearance | Off-white to brown solid powder |
| LogP | 2.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 26 |
| Complexity | 580 |
| Defined Atom Stereocenter Count | 0 |
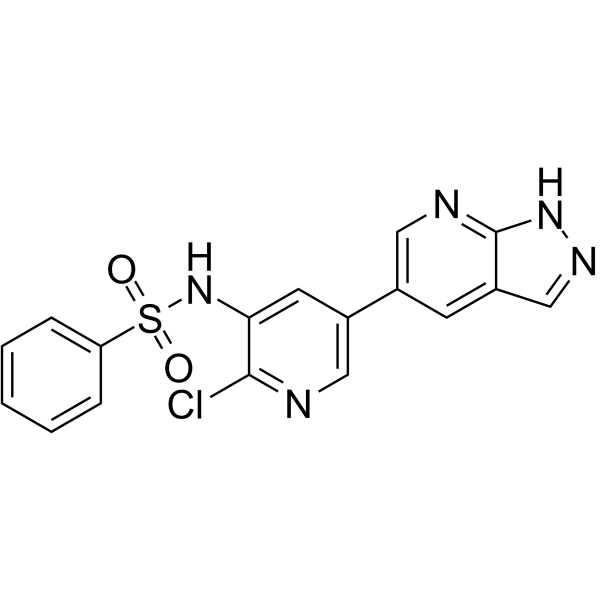
| SMILES | C1(S(NC2=CC(C3=CN=C4NN=CC4=C3)=CN=C2Cl)(=O)=O)=CC=CC=C1 |
| InChi Key | VFNUYVJFDQOTNJ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C17H12ClN5O2S/c18-16-15(23-26(24,25)14-4-2-1-3-5-14)7-12(8-19-16)11-6-13-10-21-22-17(13)20-9-11/h1-10,23H,(H,20,21,22) |
| Chemical Name | N-[2-chloro-5-(1H-pyrazolo[3,4-b]pyridin-5-yl)pyridin-3-yl]benzenesulfonamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PI3Kδ 1 nM (IC50) PI3Kα 51 nM (IC50) PI3Kβ 29 nM (IC50) PI3Kγ 37 nM (IC50) |
| ln Vitro | FD223 demonstrates significant anti-proliferative effects on the p110δ-positive AML cell lines KG-1, MOLM-16, HL-60, and EOL-1, with IC50 values of 2.82 μM, 5.82 μM, 0.87 μM, and 2.25 μM, in that order. With an IC50 value of 23.13 μM, FD223 exhibits weak anti-proliferative activity against the p110δ unexpressed MM.1R cell line[1]. The phosphorylation of Akt (Ser473) is dose-dependently reduced by FD223 (MOLM-16 cells; 0.1-5 μM; 16 hours), which is consistent with the positive control Idelalisib and shows that the PI3K/Akt pathway is blocked in MOLM-16 cells[1]. Like its positive control Idelalisib, FD223 (MOLM-16 cells; 24 hours; 1–5 μM) also stops the cell cycle at the G1 phase[1]. Cellular apoptosis is dose-dependently induced by FD223 (1–5 μM; 48 hours)[1]. |
| ln Vivo | In the MOLM-16 xenograft model, FD223 (20 and 40 mg/kg; po, per day for 14 consecutive days) exhibits strong anticancer effectiveness with a 49% tumor volume decrease at a dose of 40 mg/kg/day (po). The preliminary safety assessment of the drug indicates no appreciable toxicity[1]. Following intravenous administration, FD223 (iv; dose of 2 mg/kg; po; 10 mg/kg rats) has a moderate plasma clearance rate (C = 0.191 L·h–1·kg–1). It has good oral plasma exposures (AUC0-∞>9000 h·ng/mL), an adequate oral bioavailability (17.6%), a half-life (t1/2) of 3.74 h, and a Cmax of 1104 ng/mL in the po route[1]. |
| Cell Assay |
Apoptosis Analysis[1] Cell Types: MOLM-16 cells Tested Concentrations: 1-5 μM Incubation Duration: 48 hrs (hours) Experimental Results: Dose-dependently induced cellular apoptosis, which is superior to that of positive control Idelalisib. Western Blot Analysis[1] Cell Types: MOLM-16 cells Tested Concentrations: 0.1-5 μM Incubation Duration: 16 hrs (hours) Experimental Results: Dose-dependently decreased phosphorylation of Akt (Ser473). |
| Animal Protocol |
Animal/Disease Models: MOLM-16 xenograft model of BALB/c nude mice[1] Doses: 20 and 40 mg/kg Route of Administration: Po, per day for 14 days Experimental Results: demonstrated a dose-dependent tumor growth inhibition (TGI) of 31% for 20 mg/kg and 49% for 40 mg/kg |
| References |
[1]. Bioisosteric replacements of the indole moiety for the development of a potent and selective PI3Kδ inhibitor: Design, synthesis and biological evaluation [published online ahead of print, 2021 Jun 21]. Eur J Med Chem. 2021;223:113661. |
Solubility Data
| Solubility (In Vitro) | DMSO : 100 mg/mL (259.18 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.48 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (6.48 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5918 mL | 12.9591 mL | 25.9182 mL | |
| 5 mM | 0.5184 mL | 2.5918 mL | 5.1836 mL | |
| 10 mM | 0.2592 mL | 1.2959 mL | 2.5918 mL |