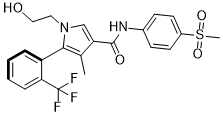
Esaxerenone, formerly known as CS-3150, XL-550, is a nonsteroidal antimineralocorticoid which was discovered by Exelixis and is now under development by Daiichi Sankyo Company for the treatment of hypertension, essential hypertension, hyperaldosteronism, and diabetic nephropathies. It acts as a highly selective silent antagonist of the mineralocorticoid receptor (MR), the receptor for aldosterone, with greater than 1,000-fold selectivity for this receptor over other steroid hormone receptors, and 4-fold and 76-fold higher affinity for the MR relative to the existing antimineralocorticoids spironolactone and eplerenone.
Physicochemical Properties
| Molecular Formula | C22H21F3N2O4S |
| Molecular Weight | 466.4752 |
| Exact Mass | 466.117 |
| CAS # | 1632006-28-0 |
| Related CAS # | 1632006-28-0;880780-76-7;1072195-82-4 (+ isomer);1072195-83-5 (- isomer); |
| PubChem CID | 25052023 |
| Appearance | White to light yellow solid powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 581.3±50.0 °C at 760 mmHg |
| Flash Point | 305.4±30.1 °C |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.581 |
| LogP | 3.15 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 32 |
| Complexity | 747 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | NOSNHVJANRODGR-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C22H21F3N2O4S/c1-14-18(21(29)26-15-7-9-16(10-8-15)32(2,30)31)13-27(11-12-28)20(14)17-5-3-4-6-19(17)22(23,24)25/h3-10,13,28H,11-12H2,1-2H3,(H,26,29) |
| Chemical Name | 1-(2-hydroxyethyl)-4-methyl-N-(4-(methylsulfonyl)phenyl)-5-(2-(trifluoromethyl)phenyl)-1H-pyrrole-3-carboxamide |
| Synonyms | Esaxerenone CS-3150 CS 3150 CS3150 XL-550 XL550 XL 550. |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vivo | The maximum plasma concentration (Cmax) and area under the plasma concentration versus time curve (AUC) increased with increasing dose after a single oral administration of 0.1, 0.3, 1, and 3 mg/kg esaxerenone. Esaxerenone's maximum plasma concentration (Tmax) can be reached in 2.0–4.5 hours. The systemic clearance (CL) and volume of distribution (Vss) at steady state following an intravenous injection of 0.1, 0.3, 1 and 3 mg/kg of esaxerenone were 3.53 to 6.69 mL/min/kg and 1.47 to 2.49 L/kg, respectively. Rats: 2.79 to 3.69 mL/min/kg; cynomolgus monkeys: 1.34 to 1.54 L/kg; and rats: 2.79 to 3.69 mL/min/kg. Rat urine and feces excreted 3.9% and 91.4%, respectively, of the dosed radioactive material within 168 hours of administration, for a total of 95.2%. In monkeys, 82.3% of the radioactive material expelled in feces and 11.5% in urine made up a total of 93.9% within 168 hours [1]. |
| References |
[1]. Pharmacokinetics, distribution, and disposition of esaxerenone, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist, in rats and monkeys. Xenobiotica. 2017 Dec;47(12):1090-1103. |
| Additional Infomation | Esaxerenone is under investigation in clinical trial NCT02722265 (Long-term Study of CS-3150 as Monotherapy or in Combination With Other Antihypertensive Drug in Japanese Patients With Essential Hypertension). |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~214.38 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (4.46 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (4.46 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 3: 2.08 mg/mL (4.46 mM) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1437 mL | 10.7186 mL | 21.4371 mL | |
| 5 mM | 0.4287 mL | 2.1437 mL | 4.2874 mL | |
| 10 mM | 0.2144 mL | 1.0719 mL | 2.1437 mL |