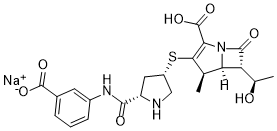
Ertapenem sodium (MK-0826; L-749345; Invanoz; Invanz), the sodium salt of ertapenem, is a 1-β-methyl carbapenem antibiotic marketed by Merck as Invanz. Ertapenem is a long-acting, broad-spectrum antibiotic of β-lactam subclass. Ertapenem has a broad spectrum of antibacterial activity including common aerobic and anaerobic bacteria and organisms with extended-spectrum β-lactamases. Ertapenem is an inhibitor of bacteria cell-wall synthesis, it acts by binding to penicillin binding proteins located on the bacterial cell wall, in particular PBPs 2 and 3, thereby inhibiting the final transpeptidation step in the synthesis of peptidoglycan, an essential component of the bacterial cell wall. Inhibition of peptidoglycan synthesis results in weakening and lysis of the cell wall and cell death. Erapenem is resistant to hydrolysis by a variety of beta-lactamases, including penicillinases, cephalosporinases and extended-spectrum beta-lactamases.
Physicochemical Properties
| Molecular Formula | C22H24N3NAO7S |
| Molecular Weight | 497.5 |
| Exact Mass | 497.123 |
| Elemental Analysis | C, 53.11; H, 4.86; N, 8.45; Na, 4.62; O, 22.51; S, 6.45 |
| CAS # | 153773-82-1 |
| Related CAS # | Ertapenem disodium;153832-38-3;Ertapenem;153832-46-3 |
| PubChem CID | 11145493 |
| Appearance | White to light yellow solid powder |
| Boiling Point | 813.9ºC at 760 mmHg |
| Flash Point | 446ºC |
| Vapour Pressure | 5.26E-28mmHg at 25°C |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 34 |
| Complexity | 882 |
| Defined Atom Stereocenter Count | 6 |
| SMILES | O=C([C@H]1NC[C@@H](SC2=C(C(O)=O)N3[C@]([C@]([C@@H](C)O)([H])C3=O)([H])[C@H]2C)C1)NC4=CC=CC(C([O-])=O)=C4.[Na+] |
| InChi Key | ZXNAQFZBWUNWJM-HRXMHBOMSA-M |
| InChi Code | InChI=1S/C22H25N3O7S.Na/c1-9-16-15(10(2)26)20(28)25(16)17(22(31)32)18(9)33-13-7-14(23-8-13)19(27)24-12-5-3-4-11(6-12)21(29)30;/h3-6,9-10,13-16,23,26H,7-8H2,1-2H3,(H,24,27)(H,29,30)(H,31,32);/q;+1/p-1/t9-,10-,13+,14+,15-,16-;/m1./s1 |
| Chemical Name | Sodium;3-[[(2S,4S)-4-[[(4R,5S,6S)-2-carboxy-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-en-3-yl]sulfanyl]pyrrolidine-2-carbonyl]amino]benzoate |
| Synonyms | MK 826; L-749345; MK-826; L749345; MK826; MK-0826; MK 0826; MK0826; L 749345; Ertapenem Sodium; Trade Name: Invanoz;Ertapenem sodium; 153773-82-1; ertapenem monosodium; Ertapenem sodium salt; UNII-2T90KE67L0; CHEBI:60070; Invanz (TN); Invanz |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam |
| ln Vitro | Ertapenem sodium (0-100 μg/mL, approximately 48 hours) exhibits activity against 99.1% of all anaerobes, with MICs for B.fragilis and B.vulgatus species being ≥8 μg/mL and 0.12 μg/mL and MIC90 of 1 μg/mL, respectively[1]. |
| ln Vivo |
In a S. aureus thigh tissue infection model, subcutaneous injection of ertapenem sodium (0–10 mg/kg, 0-120 h post-infection) reduces the organism by > 3 log10 CFU at 10 mg/kg and keeps the activity at 3.3 and 4.4 log10 CFU eliminated at 2 mg/kg[2]. In addition to being active against all gram-positive organisms, ertapenem sodium (subcutaneous injection, 4 hours after infection, systemic infection model) is also active against gram-negative organisms with ED50s of less than 0.25 mg/kg/dose[2]. |
| Cell Assay |
Cell Line: B. fragilis (ATCC 25285), B. thetaiotaomicron (ATCC 29741), and Eubacterium lentum (ATCC 43055) Concentration: 0-100 μg/mL approximately Incubation Time: 48 h Result: 98.8% of the isolates in the B. fragilis group were susceptible, and 99.1% of all isolates were inhibited with a mode MIC of 0.12 μg/mL and MIC90 of 1 μg/mL. |
| Animal Protocol |
Animal Model: S. aureus thigh tissue infection model (DBA/2 mice)[2] Dosage: 0.5,1, 2, 5, 10 mg/kg (given at 2, 6, 10, 24, 48, 72, 96, 120 h) Administration: Subcutaneous injection (0.5 mL after infection) Result:> 3 logs were displayed.10 CFU decrease in the organism when compared to controls not receiving antibiotics at a dose of 10 mg/kg. Maintained the activity with 3.3 and 4.4 log10 CFU eliminated at 2 mg/kg. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that ertapenem produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush has been reported with beta-lactams, but these effects have not been adequately evaluated. Ertapenem is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| References |
[1]. Kenneth E Aldridge. Ertapenem (MK-0826), a new carbapenem: comparative in vitro activity against clinically significant anaerobes. Diagn Microbiol Infect Dis. 2002 Oct;44(2):181-6. [2]. In vivo activity and pharmacokinetic evaluation of a novel long-acting carbapenem antibiotic, MK-826 (L-749,345). Antimicrob Agents Chemother. 1998 Aug;42(8):1996-2001. |
| Additional Infomation |
Ertapenem sodium is the monosodium salt of ertapenem. It is used for the treatment of moderate to severe susceptible infections including intra-abdominal and acute gynaecological infections, pneumonia, and infections of the skin and of the urinary tract. It is more stable to renal dehydropeptidase I tham imipenem, and so unlike imipenem, its use with cilastatin, which inhibits the enzyme, is not required. It has a role as an antibacterial drug. It contains an ertapenem(1-). Ertapenem Sodium is the sodium salt of ertapenem, a 1-beta-methyl carbapenem and a broad-spectrum beta-lactam antibiotic with bactericidal activity. Ertapenem binds to penicillin binding proteins (PBPs) located on the bacterial cell wall, in particular PBPs 2 and 3, thereby inhibiting the final transpeptidation step in the synthesis of peptidoglycan, an essential component of the bacterial cell wall. Inhibition of peptidoglycan synthesis results in weakening and lysis of the cell wall and cell death. In vitro, this agent has shown activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria. Erapenem is resistant to hydrolysis by a variety of beta-lactamases, including penicillinases, cephalosporinases and extended-spectrum beta-lactamases. A carbapenem derivative antibacterial agent that is more stable to renal dehydropeptidase I than IMIPENEM, but does not need to be given with an enzyme inhibitor such as CILASTATIN. It is used in the treatment of Gram-positive and Gram-negative bacterial infections including intra-abdominal infections, acute gynecological infections, complicated urinary tract infections, skin infections, and respiratory tract infections. It is also used to prevent infection in colorectal surgery. See also: Ertapenem Sodium (preferred); Ertapenem (has active moiety). Drug Indication TreatmentErtapenem SUN is indicated in paediatric patients (3 months to 17 years of age) and in adults for the treatment of the following infections when caused by bacteria known or very likely to be susceptible to ertapenem and when parenteral therapy is required (see sections 4. 4 and 5. 1): - Intra-abdominal infections- Community acquired pneumonia- Acute gynaecological infections- Diabetic foot infections of the skin and soft tissue (see section 4. 4)PreventionErtapenem SUN is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery (see section 4. 4). Consideration should be given to official guidance on the appropriate use of antibacterial agents. TreatmentTreatment of the following infections when caused by bacteria known or very likely to be susceptible to ertapenem and when parenteral therapy is required: intra-abdominal infections; community-acquired pneumonia; acute gynaecological infections; diabetic foot infections of the skin and soft tissue. PreventionInvanz is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery. Consideration should be given to official guidance on the appropriate use of antibacterial agents. |
Solubility Data
| Solubility (In Vitro) |
DMSO : ~100 mg/mL ( ~201.0 mM O) Water : 50~100 mg/mL(~100.50 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 100 mg/mL (201.01 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0101 mL | 10.0503 mL | 20.1005 mL | |
| 5 mM | 0.4020 mL | 2.0101 mL | 4.0201 mL | |
| 10 mM | 0.2010 mL | 1.0050 mL | 2.0101 mL |