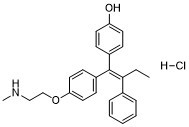
Endoxifen HCl (Z-Endoxifen; N-Desmethyl-4-hydroxytamoxifen), the hydrochloride salt of endoxifen which is an active metabolite of Tamoxifen, is a potent and selective estrogen receptor antagonist/SERM with potential anticancer activity. It has been found to be effective in patients that have failed previous hormonal therapies. Endoxifen shows anti-estrogenic effects, and decreases the E2-induced PR expression in MCF-7 cells. Endoxifen also blocks ER-alpha transcriptional activity and inhibits estrogen-induced breast cancer cell proliferation. In MCF7, HS 578T, and BT-549 cells, Endoxifen significantly inhibits cell proliferation. Endoxifen also exhibits four-fold higher inhibition on PKC activity compared to tamoxifen.
Physicochemical Properties
| Molecular Formula | C25H28CLNO2 | |
| Molecular Weight | 409.95 | |
| Exact Mass | 409.18 | |
| CAS # | 1032008-74-4 | |
| Related CAS # | Endoxifen (Z-isomer);112093-28-4;Endoxifen hydrochloride;1197194-41-4;Endoxifen (E-isomer);114828-90-9;Endoxifen;110025-28-0;Endoxifen E-isomer hydrochloride;1197194-61-8 | |
| PubChem CID | 54613017 | |
| Appearance | White to off-white solid powder | |
| LogP | 6.552 | |
| Hydrogen Bond Donor Count | 3 | |
| Hydrogen Bond Acceptor Count | 3 | |
| Rotatable Bond Count | 8 | |
| Heavy Atom Count | 29 | |
| Complexity | 467 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | CC/C(=C(\C1=CC=C(C=C1)O)/C2=CC=C(C=C2)OCCNC)/C3=CC=CC=C3.Cl |
|
| InChi Key | RPFIMPDXTABYCN-BJFQDICYSA- | |
| InChi Code | InChI=1S/C25H27NO2.ClH/c1-3-24(19-7-5-4-6-8-19)25(20-9-13-22(27)14-10-20)21-11-15-23(16-12-21)28-18-17-26-2;/h4-16,26-27H,3,17-18H2,1-2H3;1H/b25-24- | |
| Chemical Name | (Z)-4-(1-(4-(2-(methylamino)ethoxy)phenyl)-2-phenylbut-1-en-1-yl)phenol hydrochloride | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro |
|
||
| ln Vivo |
|
||
| Animal Protocol |
|
||
| References | Cancer Chemother Pharmacol.2005 May;55(5):471-8;Breast Cancer Res Treat.2010Jul;122(2):579-84. | ||
| Additional Infomation | Endoxifen Hydrochloride is the hydrochloride salt and the z (cis-) stereoisomer of endoxifen with potential antineoplastic activity. Endoxifen, the active metabolite of tamoxifen, competitively inhibits the binding of estradiol to estrogen receptors, thereby preventing the receptor from binding to the estrogen-response element on DNA and thus reducing DNA synthesis. Unlike tamoxifen, however, which relies on CYP2D6 activity for its conversion to the active metabolite endoxifen, the direct administration of endoxifen bypasses the CYP2D6 route. As CYP2D6 activity can vary widely among individuals due to genetic CYP2D6 polymorphisms, endoxifen is therefore theoretically more potent and more uniform in its bioavailability across patient populations. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (6.10 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (6.10 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 1.25 mg/mL (3.05 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 12.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4393 mL | 12.1966 mL | 24.3932 mL | |
| 5 mM | 0.4879 mL | 2.4393 mL | 4.8786 mL | |
| 10 mM | 0.2439 mL | 1.2197 mL | 2.4393 mL |