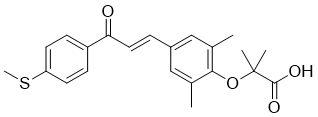
Elafibranor (formerly GFT505) is a dual agonist of the PPARα/δ (peroxisome proliferator-activated receptor-α and -δ) with EC50 values of 45 and 175 nM, respectively. In addition to lowering inflammation, it can enhance insulin sensitivity, glucose homeostasis, and lipid metabolism. The treatment of T2DM, non-alcoholic fatty liver disease, insulin resistance, dyslipidemia, and other cardiometabolic disorders is currently being pursued with elafibranor. Both elafibranor and its active metabolite, GFT1007, exhibit strong agonist activity for PPAR-α and somewhat for PPAR-δ.
Physicochemical Properties
| Molecular Formula | C22H24O4S | |
| Molecular Weight | 384.49 | |
| Exact Mass | 384.139 | |
| Elemental Analysis | C, 68.73; H, 6.29; O, 16.64; S, 8.34 | |
| CAS # | 923978-27-2 | |
| Related CAS # | 824932-88-9; 923978-27-2; | |
| PubChem CID | 9864881 | |
| Appearance | Light yellow to yellow solid powder | |
| Density | 1.2±0.1 g/cm3 | |
| Boiling Point | 569.0±50.0 °C at 760 mmHg | |
| Flash Point | 297.9±30.1 °C | |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C | |
| Index of Refraction | 1.606 | |
| LogP | 5.63 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 27 | |
| Complexity | 537 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | O(C1C(C)=CC(/C=C/C(C2C=CC(SC)=CC=2)=O)=CC=1C)C(C)(C)C(=O)O |
|
| InChi Key | AFLFKFHDSCQHOL-IZZDOVSWSA-N | |
| InChi Code | InChI=1S/C22H24O4S/c1-14-12-16(13-15(2)20(14)26-22(3,4)21(24)25)6-11-19(23)17-7-9-18(27-5)10-8-17/h6-13H,1-5H3,(H,24,25)/b11-6+ | |
| Chemical Name | 2-[2,6-dimethyl-4-[(E)-3-(4-methylsulfanylphenyl)-3-oxoprop-1-enyl]phenoxy]-2-methylpropanoic acid | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PPAR-α (IC50 = 45 nM); PPAR-δ (IC50 = 175 nM) |
| ln Vitro | Elafibranor (GFT505) is being developed as a dual agonist for PPAR-α and PPAR-δ to inhibit non-alcoholic fatty liver disease and type 2 diabetes. GFT1007, an active metabolite of elafibranor, exhibits strong agonist activity for PPAR-α and somewhat for PPAR-δ[1]. |
| ln Vivo |
Elafibranor is well tolerated; however, it does result in a slight, reversible elevation in serum creatinine and neither weight gain nor cardiac events. In addition to lowering inflammation, elafibranor enhances lipid metabolism and glucose homeostasis[2]. Treatment with elafibranor (GFT505) improves plasma lipids and glucose regulation in diabetic db/db mice. With Elafibranor, hepatic expression of the major gluconeogenic enzymes fructose 1,6-bisphosphatase 1 (FBP1), PEPCK, and glucose 6-phosphatase (G6Pase) is significantly dose-dependently reduced. In monkeys, PPARγ-activating agonists do not cause cardiac side effects when elabranor is used[3].
In intention-to-treat analysis, there was no significant difference between the elafibranor and placebo groups in the protocol-defined primary outcome. However, NASH resolved without fibrosis worsening in a higher proportion of patients in the 120-mg elafibranor group vs the placebo group (19% vs 12%; odds ratio = 2.31; 95% confidence interval: 1.02-5.24; P = .045), based on a post-hoc analysis for the modified definition. In post-hoc analyses of patients with nonalcoholic fatty liver disease activity score ≥4 (n = 234), elafibranor 120 mg resolved NASH in larger proportions of patients than placebo based on the protocol definition (20% vs 11%; odds ratio = 3.16; 95% confidence interval: 1.22-8.13; P = .018) and the modified definitions (19% vs 9%; odds ratio = 3.52; 95% confidence interval: 1.32-9.40; P = .013). Patients with NASH resolution after receiving elafibranor 120 mg had reduced liver fibrosis stages compared with those without NASH resolution (mean reduction of 0.65 ± 0.61 in responders for the primary outcome vs an increase of 0.10 ± 0.98 in nonresponders; P < .001). Liver enzymes, lipids, glucose profiles, and markers of systemic inflammation were significantly reduced in the elafibranor 120-mg group vs the placebo group. Elafibranor was well tolerated and did not cause weight gain or cardiac events, but did produce a mild, reversible increase in serum creatinine (effect size vs placebo: increase of 4.31 ± 1.19 μmol/L; P < .001). Conclusions: A post-hoc analysis of data from trial of patients with NASH showed that elafibranor (120 mg/d for 1 year) resolved NASH without fibrosis worsening, based on a modified definition, in the intention-to-treat analysis and in patients with moderate or severe NASH. However, the predefined end point was not met in the intention to treat population. Elafibranor was well tolerated and improved patients' cardiometabolic risk profile. ClinicalTrials.gov number: NCT01694849.[2] Elafibranor attenuated in vitro key features of NASH, and dramatically lowered lipid load as well as the expression and secretion of inflammatory chemokines, which in vivo are responsible for the recruitment of immune cells. This reduction in inflammatory response was NFκB-mediated. In summary, this human-relevant, in vitro system proved to be a sensitive testing tool for the investigation of novel anti-NASH compounds.[4] |
| Enzyme Assay | Elafibranor is an agonist of PPARα/δ with EC50 values of 45 and 175 nM, in that order. As a dual PPAR-α/PPAR-δ agonist, GFT505 is being developed to treat non-alcoholic fatty liver disease and type 2 diabetes. GThe active metabolite of GFT505, GFT1007, exhibits strong agonist activity for PPAR-α and to a lesser degree for PPAR-δ. |
| Cell Assay |
hSKP-HPC cell culture and establishment of steatosis and NASH in vitro models [4] hSKP were isolated from circumcision samples of young boys from 1 to 10 years old, after informed consent from the parents and approval of the medical ethical committee of the UZ Brussels. These cells were cultured and differentiated to hepatocyte-like cells (hSKP-HPC) as previously documented. Steatosis was mimicked in vitro by exposing hSKP-HPC (24 h) to insulin (100 nM) and glucose (4.5 mg/mL). NASH conditions were created by concurrent exposure (24 h) to sodium oleate (65 μM) and palmitic acid (45 μM) in combination with a pro-inflammatory (50 ng/mL tumour necrosis factor (TNF)-alpha + 25 ng/mL interleukin (IL)-1β) and pro-fibrotic (8 ng/mL transforming growth factor (TGF)-β1) cytokine cocktail. Sodium oleate was complexed with 7% (w/v) bovine serum albumin (BSA) fatty acid-free. Palmitic acid and elafibranor were dissolved in dimethyl sulfoxide (DMSO). Cells were concomitantly exposed to NASH triggers and elafibranor (10 μM and 30 μM). Final concentrations of BSA and DMSO in all samples were 0.14% (w/v) and 0.15% (v/v), respectively (except for the determination of the sodium oleate and palmitic acid concentration (shown in Supplementary Fig. 1A and B), where 1.4% (w/v) BSA and 0.5% (v/v) DMSO were used, respectively). Antibody array for determination of cytokines and chemokines [4] Fresh cell culture media of control samples and cells exposed to NASH inducers and elafibranor (10 μM and 30 μM) were collected for determining interleukin and chemokine secretion using a human cytokine antibody array (120 Targets) according to the manufacturer's protocol. Visualization of the hybridization spots was performed using a Chemidoc™ MP system. Image Lab 5.0 software was used for semi-quantitative data analysis (n = 5, except for hSKP-HPC NASH +elafibranor 30 μM: n = 3). |
| Animal Protocol |
hApoE2 KI and hApoE2 KI/PPAR-α KO mice 30 mg/kg oral gavage Researchers report here the efficacy and safety of GFT505, a novel liver-targeted peroxisome proliferator-activated receptor alpha/delta (PPARα/δ) agonist, in the db/db mouse model of diabetes. Mice were treated with vehicle, GFT505, PPARγ agonist rosiglitazone or dual-PPARα/γ agonist aleglitazar for up to 8 weeks. All compounds comparably reduced fasting glycaemia and HbA1c and improved insulin sensitivity. The glucose-lowering effect of GFT505 was associated with decreased hepatic gluconeogenesis, correlating with reduced expression of gluconeogenic genes. In contrast with the PPARγ-activating drugs, treatment with GFT505 did not affect heart weight and did not increase plasma adiponectin concentrations. This absence of cardiac effects of GFT505 was confirmed after 12 months of administration in cynomolgus monkeys, by the absence of echocardiographic and histological findings. Moreover, long-term GFT505 administration to monkeys induced no change in haematological parameters or in bone marrow differential cell counts. Compared to PPARγ-activating drugs, the dual-PPARα/δ agonist GFT505 therefore shows an improved benefit/risk ratio, treating multiple features of type 2 diabetes without inducing the cardiac side-effects associated with PPARγ activation.[3] Patients with NASH without cirrhosis were randomly assigned to groups given elafibranor 80 mg (n = 93), elafibranor 120 mg (n = 91), or placebo (n = 92) each day for 52 weeks at sites in Europe and the United States. Clinical and laboratory evaluations were performed every 2 months during this 1-year period. Liver biopsies were then collected and patients were assessed 3 months later. The primary outcome was resolution of NASH without fibrosis worsening, using protocol-defined and modified definitions. Data from the groups given the different doses of elafibranor were compared with those from the placebo group using step-down logistic regression, adjusting for baseline nonalcoholic fatty liver disease activity score.[2] |
| Toxicity/Toxicokinetics |
Hepatotoxicity In preregistration clinical trials, elafibranor was found to decrease both serum aminotransferase and alkaline phosphatase elevations in a high proportion of patients with PBC. In preliminary dose-finding studies in healthy volunteers, elevations of ALT and AST levels above 5 times the upper limit of normal (ULN) were found to be dose related and occurred in one-third of subjects exposed to doses above 120 mg daily. In contrast, in clinical trials of elafibranor in doses of 80 mg daily in patients with NASH and PBC, ALT elevations above 5 times ULN occurred in only 1% to 2% of patients, typically arising within the first few months of therapy and resolving spontaneously without drug interruption and without jaundice or symptoms. Careful assessment of cases with ALT elevations concluded that 3 were possibly due to drug induced injury, 2 among 138 patients with PBC and 1 among 1433 patients with NASH. Among patients with myalgia and CPK elevations during elafibranor therapy, one patient with preexisting cirrhosis who was also taking a statin, developed jaundice [5.5 mg/dL] with elevations in ALT [300 U/L] and AST [828 U/L] concurrent with rhabdomyolysis [CPK 12,647 U/L] and subsequently suffered hepatic decompensation. The incidence of gallstones and cholecystitis also may be increased with elafibranor therapy. Rare instances of drug induced liver injury are known to occur with other PPARα (fenofibrate, bezafibrate) and PPARγ (pioglitazone, rosiglitazone) agonists. Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury). |
| References |
[1]. Early investigational drugs targeting PPAR-α for the treatment of metabolic disease.Expert Opin Investig Drugs. 2015 May;24(5):611-21. [2]. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016 May;150(5):1147-1159. [3]. The dual peroxisome proliferator-activated receptor alpha/delta agonist GFT505 exerts anti-diabetic effects in db/db mice without peroxisome proliferator-activated receptor gamma-associated adverse cardiac effects. Diab Vasc Dis Res. 2014 Nov;11(6):440-7. [4]. Elafibranor restricts lipogenic and inflammatory responses in a human skin stem cell-derived model of NASH. Pharmacol Res. 2019 Jun:144:377-389. |
| Additional Infomation |
Elafibranor (code name GFT505) is a multimodal and pluripotent medication for treatment of atherogenic dyslipidemia for an overweight patient with or without diabetes. It is an oral treatment that acts on the 3 sub-types of PPAR (PPARa, PPARg, PPARd) with a preferential action on PPARa. As of February 2016, elafibranor has completed 8 clinical trials and a phase III is in progress. Elafibranor is an orally available peroxisome proliferator-activated receptor agonist that is used in combination with ursodeoxycholic acid to treat primary biliary cholangitis. Elafibranor therapy is associated with rare instances of worsening of liver enzymes during therapy but has not been convincingly linked to episodes of clinically apparent liver injury with jaundice. Drug Indication Investigated for use/treatment in atherosclerosis and diabetes mellitus type 2. Treatment of primary biliary cholangitis Treatment of non-alcoholic fatty liver disease (NAFLD) including non-alcoholic steatohepatitis (NASH) Mechanism of Action GFT505 is an oral treatment that acts on the 3 sub-types of PPAR (PPARa, PPARg, PPARd) with a preferential action on PPARa. It has a sophisticated mechanism of action. It is able to differentially recruit cofactors to the nuclear receptor, which subsequently lead to differential regulation of genes and biological effect. Therefore, the ability to identify and profile the activity of selective nuclear receptor modulator (SNuRMs) is a powerful approach to select innovative drug candidates with improved efficacy and diminished side effects. These pluripotent and multimodal molecules have significant positive effects on obesity, insulin-resistance and diabetes, atherosclerosis, inflammation, and the lipid triad (increasing of HDL cholesterol, lowering of triglycerides and LDL cholesterol). Introduction: The fibrates have been used for many years to treat dyslipidemias and have also recently been shown to have anti-inflammatory effects. They are relatively weak PPAR-α agonists and do have some adverse effects. Novel compounds are in development, which are selective PPAR modulators (SPPARMs) and have more potent PPAR-α agonist activity. These may prove to have advantages in the treatment of dyslipidemia, insulin resistance and non-alcoholic fatty liver disease (NAFLD). Areas covered: This review focuses on PPAR-α agonists or SPPARMs in development describing the preclinical and early clinical studies. The information was obtained by searching the published literature and abstracts from recent meetings. Ongoing clinical trials were identified using the Clinicaltrial.gov database. Expert opinion: There is still a need for new drugs to treat atherogenic dyslipidemia. The highly potent and selective PPAR-α agonist K-877 has shown beneficial effects on atherogenic dyslipidemia and absence of some adverse effects seen with fibrates. The dual PPAR-α/PPAR-δ agonist GFT-505 has shown favorable results in improving atherogenic dyslipidemia and insulin resistance and appears to be a potential candidate for the treatment of NAFLD. Long-term trials are needed to assess the safety and efficacy of these new agents for cardiovascular and liver outcomes.[1] Non-alcoholic steatohepatitis (NASH) is characterized by hepatocellular steatosis with concomitant hepatic inflammation. Despite its pandemic proportions, no anti-NASH drugs have been approved yet. This is partially because drug development is decelerated due to the lack of adequate tools to assess the efficacy of potential new drug candidates. The present study describes the development and application of a new preclinical model for NASH using hepatic cells generated from human skin-derived precursors. Exposure of these cells to lipogenic (insulin, glucose, fatty acids) and pro-inflammatory factors (IL-1β, TNF-α, TGF-β) resulted in a characteristic NASH response, as indicated by intracellular lipid accumulation, modulation of NASH-specific gene expression, increased caspase-3/7 activity and the expression and/or secretion of inflammatory markers, including CCL2, CCL5, CCL7, CCL8, CXCL5, CXCL8, IL1a, IL6 and IL11. The human relevance of the proposed NASH model was verified by transcriptomics analyses that revealed commonly modulated genes and the identification of the same gene classes between the in vitro system and patients suffering from NASH. The application potential of this in vitro model was demonstrated by testing elafibranor, a promising anti-NASH compound currently under clinical phase III trial evaluation. Elafibranor attenuated in vitro key features of NASH, and dramatically lowered lipid load as well as the expression and secretion of inflammatory chemokines, which in vivo are responsible for the recruitment of immune cells. This reduction in inflammatory response was NFκB-mediated. In summary, this human-relevant, in vitro system proved to be a sensitive testing tool for the investigation of novel anti-NASH compounds.[4] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.87 mg/mL (7.46 mM) (saturation unknown) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.17 mg/mL (5.64 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 21.7 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 3: ≥ 2.17 mg/mL (5.64 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 21.7 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 4: ≥ 2.17 mg/mL (5.64 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 21.7 mg/mL clear DMSO stock solution to 900 μL corn oil and mix evenly. Solubility in Formulation 5: ≥ 2.17 mg/mL (5.64 mM)(saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one),clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 21.7 mg/mL clear DMSO stock solution to 900 μL corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6008 mL | 13.0042 mL | 26.0085 mL | |
| 5 mM | 0.5202 mL | 2.6008 mL | 5.2017 mL | |
| 10 mM | 0.2601 mL | 1.3004 mL | 2.6008 mL |