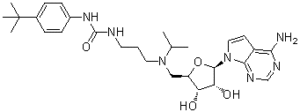
Description: EPZ004777 (EPZ-004777) is a potent and selective inhibitor of DOT1 Like Histone Lysine Methyltransferase (DOT1L) inhibitor with antineoplastic activity. It inhibits DOT1L with an IC50 of 0.4 nM in a cell-free assay. It shows potent in vitro antiproliferative activity and high in vivo antitumor efficacy. Exposure of leukemic cells to EPZ 004777 laed to selective killing of cells bearing MLL gene translocation, with little effect on non-MLL-translocated cells. In addition, EPZ004777 extended the survival of mice bearing a MLL xenograft model.
Physicochemical Properties
| Molecular Formula | C28H41N7O4 | |
| Molecular Weight | 539.67 | |
| Exact Mass | 539.322 | |
| CAS # | 1338466-77-5 | |
| Related CAS # | EPZ004777 hydrochloride;1380316-03-9 | |
| PubChem CID | 56962336 | |
| Appearance | White to off-white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 740.7±60.0 °C at 760 mmHg | |
| Flash Point | 401.7±32.9 °C | |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C | |
| Index of Refraction | 1.646 | |
| LogP | 3.94 | |
| Hydrogen Bond Donor Count | 5 | |
| Hydrogen Bond Acceptor Count | 8 | |
| Rotatable Bond Count | 10 | |
| Heavy Atom Count | 39 | |
| Complexity | 788 | |
| Defined Atom Stereocenter Count | 4 | |
| SMILES | CC(C)N(CCCNC(=O)NC1=CC=C(C=C1)C(C)(C)C)C[C@@H]2[C@H]([C@H]([C@@H](O2)N3C=CC4=C(N=CN=C43)N)O)O |
|
| InChi Key | WXRGFPHDRFQODR-ICLZECGLSA-N | |
| InChi Code | InChI=1S/C28H41N7O4/c1-17(2)34(13-6-12-30-27(38)33-19-9-7-18(8-10-19)28(3,4)5)15-21-22(36)23(37)26(39-21)35-14-11-20-24(29)31-16-32-25(20)35/h7-11,14,16-17,21-23,26,36-37H,6,12-13,15H2,1-5H3,(H2,29,31,32)(H2,30,33,38)/t21-,22-,23-,26-/m1/s1 | |
| Chemical Name | 1-(3-((((2R,3S,4R,5R)-5-(4-amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(isopropyl)amino)propyl)-3-(4-(tert-butyl)phenyl)urea | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | EPZ004777 shows a strong, concentration-dependent inhibition of 400±100 pM of DOT1L enzyme activity. When compared to other HMTs (PRMT5, 521±137 nM; others, >50 μM), EPZ004777 shows a notable degree of selectivity for DOT1L inhibition. The effects of prolonged EPZ004777 therapy were notably unique to cell lines having MLL rearrangements. While Jurkat cell proliferation remained unaffected, EPZ004777 dramatically decreased the number of viable MV4-11 and MOLM-13 cells. While MV4-11 cells in the presence of EPZ004777 continued to divide, a small population of these cells remained viable; yet, when tracking their growth curve over time, their numbers remained constant. MLL-AF9 transformed cells are substantially inhibited from proliferating when exposed to doses of EPZ004777 of 3 μM or above [1]. MLL-AF10 and CALM-AF10-transformed mouse bone marrow cells are selectively inhibited from proliferating by EPZ004777 [2]. | ||
| ln Vivo | There is no obvious toxicity seen with EPZ004777, and it is well tolerated. Following a continuous 14-day exposure to EPZ004777, a complete blood count study demonstrated a statistically significant rise in the total white blood cell count, attributed to an increase in neutrophils, monocytes, and lymphocytes. Administration of EPZ004777 (50, 100, or 150 mg/mL) is well tolerated, and no appreciable weight loss is seen[1]. | ||
| Animal Protocol |
|
||
| References |
[1]. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell. 2011 Jul 12;20(1):53-65. [2]. Abrogation of MLL-AF10 and CALM-AF10-mediated transformation through genetic inactivation or pharmacological inhibition of the H3K79 methyltransferase Dot1l. Leukemia. 2013 Apr;27(4):813-22. |
||
| Additional Infomation | 1-[3-[[(2R,3S,4R,5R)-5-(4-amino-7-pyrrolo[2,3-d]pyrimidinyl)-3,4-dihydroxy-2-oxolanyl]methyl-propan-2-ylamino]propyl]-3-(4-tert-butylphenyl)urea is a N-glycosyl compound. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 3 mg/mL (5.56 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 3 mg/mL (5.56 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 3 mg/mL (5.56 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8530 mL | 9.2649 mL | 18.5298 mL | |
| 5 mM | 0.3706 mL | 1.8530 mL | 3.7060 mL | |
| 10 mM | 0.1853 mL | 0.9265 mL | 1.8530 mL |