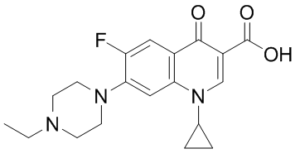
Enrofloxacin (BAY-Vp2674; PD160788; trade name Baytril) is a potent fluoroquinolone antibiotic with an MIC90 of 0.312 μg/mL for Mycoplasma bovis. Enrofloxacin is developed by the Bayer group. Enrofloxacin is currently approved by the FDA for the treatment of individual pets and domestic animals in the United States. Enrofloxacin is a broad-spectrum bactericidal antibiotic. Although the mechanism of action is not wellunderstood, Enrofloxacin is effective against a broad spectrum of gram-positive and gram-negativebacteria including most species of the following: Pseudomonas aeruginosa, Klebsiella, E.coli., Enterobacter, Campylobacter, Shigella, Salmonella, Aeromonas, Haemophilus, Proteus, Yersinia, Serratia, Vibrio, Brucella, Chlamydia, Staphylococci (including some methicillin resistant strains).
Physicochemical Properties
| Molecular Formula | C19H22FN3O3 |
| Molecular Weight | 359.3947 |
| Exact Mass | 359.164 |
| Elemental Analysis | C, 63.50; H, 6.17; F, 5.29; N, 11.69; O, 13.36 |
| CAS # | 93106-60-6 |
| Related CAS # | Enrofloxacin monohydrochloride;93106-59-3;Enrofloxacin-d5;1173021-92-5 |
| PubChem CID | 71188 |
| Appearance | Light yellow to yellow solid powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 560.5±50.0 °C at 760 mmHg |
| Melting Point | 225 °C |
| Flash Point | 292.8±30.1 °C |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.634 |
| LogP | 1.88 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 26 |
| Complexity | 613 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=C(C1C(=O)C2C(=CC(N3CCN(CC)CC3)=C(C=2)F)N(C2CC2)C=1)O |
| InChi Key | SPFYMRJSYKOXGV-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C19H22FN3O3/c1-2-21-5-7-22(8-6-21)17-10-16-13(9-15(17)20)18(24)14(19(25)26)11-23(16)12-3-4-12/h9-12H,2-8H2,1H3,(H,25,26) |
| Chemical Name | 3-Quinolinecarboxylic acid, 1,4-dihydro-1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-4-oxo-, hydrochloride |
| Synonyms | Baytril; Enrofloxacine; CFPQ; Bay-Vp-2674; BAY-Vp2674; PD 160788; BAY-Vp2674; PD160788; BAY-Vp 2674; PD-160788; endrofloxicin. |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Quinolone |
| ln Vitro | Cattle suffering from pneumonia, mastitis, arthritis, and other symptoms can be caused by the global pathogen Mycoplasma bovis. Within the tested group of fluoroquinolones, the Hungarian strains' profiles of antibiotic susceptibility are consistent. Enrofloxacin inhibits three isolates (MYC44, MYC45, and MYC46) with MIC values ≥10 μg/mL, while the remaining strains have MICs ≤0.312 or 0.625 μg/mL[1]. |
| ln Vivo | Eighty mice are subjected to a sixty-minute period of transient middle cerebral artery occlusion (MCAo), followed by reperfusion. Following MCAo, animals are randomized to receive a therapeutic medication (n=25; Enrofloxacin) upon diagnosis of lung infection, or a daily preventive medication (n=26; Enrofloxacin) beginning on the day of MCAo. Following the onset of clinical signs (general health score greater than 6), which typically occur between days 4 and 6, standard treatment was initiated right away. When compared to placebo treatment, both preventive and conventional antibiotic treatments utilizing enrofloxacin increase survival in a comparable manner[2]. |
| Animal Protocol |
Mice [2] Male C57Bl6/J mice aged 11–14 weeks are utilized. Over the course of seven days, animals treated with antibiotics receive a daily oral dose of 10 mg/kg body weight administered via feeding needle every 12 hours. Enrofloxacin (2.5% oral solution) is dispensed in saline (2 mg/mL). In contrast, animals receiving a placebo receive the same amount of saline via feeding needle. After emerging from reperfusion anesthesia, the animals in the preventive antibiotic group were given Enrofloxacin (about an hour after the procedure). Therapeutic antibiotic treatment is started as soon as clinical symptoms (general health score >5) appear and an MRI confirms the lung infection (signal rate ≥5%). Allocation to groups is done at random[2]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Pharmacokinetics and bioavailability of enrofloxacin were determined after single intravenous (IV) and intramuscular (IM) administrations of 5 mg/kg body weight (BW) to 5 healthy adult Angora goats. Plasma enrofloxacin concentrations were measured by high performance liquid chromatography. Pharmacokinetics were best described by a 2-compartment open model. The elimination half-life and volume of distribution after IV and IM administrations were similar (t1/2beta, 4.0 to 4.7 hr and Vd(ss),1.2 to 1.5 L/kg, respectively). Enrofloxacin was rapidly (t1/2a, 0.25 hr) and almost completely absorbed (F, 90%) after IM administration. Mean plasma concentrations of enrofloxacin at 24 hr after IV and IM administration (0.07 and 0.09 microg/mL, respectively) were higher than the minimal inhibitory concentration (MIC) values for most pathogens. In conclusion, once-daily IV and IM administration of enrofloxacin (5 mg/kg BW) in Angora goats may be useful in treatment of infectious diseases caused by sensitive pathogens. Plasma, urine, and skin drug concentrations were determined for dogs (n=12) given five daily oral doses of marbofloxacin (MAR) (2.75 mg/kg), enrofloxacin (ENR) (5.0 mg/kg) or difloxacin (DIF) (5.0 mg/kg). Concentrations of the active metabolite of ENR, ciprofloxacin (CIP), were also determined. The three-period, three-treatment crossover experimental design included a 21-day washout period between treatments. Area under the plasma drug concentration vs. time curve (AUC0-last, microg/mlxhr of MAR was greater than for ENR, CIP, ENR/CIP combined, and DIF. Maximum concentration (Cmax) of MAR was greater than ENR, CIP, and DIF. Time of maximum plasma concentration (Tmax) was similar for MAR and DIF; Tmax occurred earlier for ENR and later for CIP. Plasma half-life (t1/2) of MAR was longer than for ENR, CIP, and DIF. Urine concentrations of DIF were less than MAR or ENR/CIP combined, but urine concentrations of MAR and ENR/CIP combined did not differ. DIF skin concentrations were less than the concentrations of MAR or ENR/CIP combined 2 h after dosing, but skin concentrations of MAR and ENR/CIP combined did not differ. Serum concentrations and pharmacokinetics of enrofloxacin were studied in 6 mares after intravenous (IV) and intragastric (IG) administration at a single dose rate of 7.5 mg/kg body weight. In experiment 1, an injectable formulation of enrofloxacin (100 mg/ml) was given IV. At 5 min after injection, mean serum concentration was 9.04 microg/mL and decreased to 0.09 microg/mL by 24 hr. Elimination half-life was 5.33 +/- 1.05 hr and the area under the serum concentration vs time curve (AUC) was 21.03 +/- 5.19 mg x hr/L. In experiment 2, the same injectable formulation was given IG. The mean peak serum concentration was 0.94 +/- 0.97 microg/ml at 4 hr after administration and declined to 0.29 +/- 0.12 microg/ml by 24 hr. Absorption of this enrofloxacin preparation after IG administration was highly variable, and for this reason, pharmacokinetic values for each mare could not be determined. In experiment 3, a poultry formulation (32.3 mg/ml) was given IG. The mean peak serum concentration was 1.85 +/- 1.47 microg/ml at 45 min after administration and declined to 0.19 +/- 0.06 microg/mL by 24 h. Elimination half-life was 10.62 +/- 5.33 h and AUC was 16.30 +/- 4.69 mg x h/L. Bioavailability was calculated at 78.29 +/- 16.55%. Minimum inhibitory concentrations of enrofloxacin were determined for equine bacterial culture specimens submitted to the microbiology laboratory over an 11-month period. The minimum inhibitory concentration of enrofloxacin required to inhibit 90% of isolates (MIC90) was 0.25 microg/ml for Staphylococcus aureus, Escherichia coli, Salmonella spp., Klebsiella spp., and Pasteurella spp. The poultry formulation was well tolerated and could be potentially useful in the treatment of susceptible bacterial infections in adult horses. The injectable enrofloxacin solution should not be used orally. Concentrations of enrofloxacin equivalent activity were determined by microbiological assay in the plasma of healthy and E. coli-infected broilers following single intravenous and oral administrations at 10 mg/kg. Tissue distribution and residue-depletion following multiple oral doses (10 mg/kg for 3 successive days) were investigated. Pharmacokinetic variables were determined using compartmental and non-compartmental analytical methods. Plasma enrofloxacin concentrations after intravenous dosing to healthy and infected birds were best described by a two-compartments model. Enrofloxacin concentrations in plasma of infected birds were lower than those of healthy ones. The disposition kinetics of intravenously administered drug in healthy and infected birds were somewhat different. The elimination half-life (t1/2 beta) was 4.75 vs. 3.63 hr; mean residence time (MRT) was 6.72 vs 4.90 hr; apparent volume of the central compartment (Vc) was 1.11 vs 1.57 l/kg; rate constant for transfer from peripheral to central compartment (k21) was 1.15 vs 1.41 hr-1 and total body clearance (ClB) was 0.35 vs 0.53 l/hr/kg in healthy and infected birds, respectively. After oral administration, the absorption half-life (t1/2abs) in the infected birds was significantly longer than in healthy birds, while elimination half-life (t1/2el) and MRT were significantly shorter. Bioavailability was higher in infected birds (72.50%) as compared to healthy ones (69.78%). Enrofloxacin was detected in the tissues of healthy and infected birds after daily oral dosing of 10 mg/kg for 3 days. It was more concentrated in liver, kidney, and breast muscle. The minimal inhibitory concentration (MIC) of enrofloxacin against E. coli was 0.064 microgram/ml. On the basis of maintaining enrofloxacin plasma concentrations over the MIC, a dose of 10 mg/kg given intravenously every 20.14 hr or orally every 20.86 hr should provide tissue concentrations effective against E. coli infection in chickens. For more Absorption, Distribution and Excretion (Complete) data for ENROFLOXACIN (6 total), please visit the HSDB record page. Metabolism / Metabolites The pharmacokinetics of enrofloxacin and its active metabolite ciprofloxacin were investigated in goats after a single intramuscular administration of enrofloxacin at 2.5 mg/kg body weight. The plasma concentrations of enrofloxacin and ciprofloxacin were determined simultaneously by a HPLC method. The peak concentrations (Cmax) of enrofloxacin (1.13 microg/ml) and ciprofloxacin (0.24 microg/ml) were observed at 0.8 and 1.2 hr, respectively. The elimination half-life (t1/2beta), volume of distribution (Vd(area)), total body clearance (Cl(B)) and mean residence time (MRT) of enrofloxacin were 0.74 hr, 1.42 l/kg, 1329 ml/hr per kg and 1.54 hr, respectively. The t1/2beta, area under the plasma concentration-time curve (AUC) and the MRT of ciprofloxacin were 1.38 h, 0.74 microg h/ml and 2.73 h, respectively. The metabolic conversion of enrofloxacin to ciprofloxacin was appreciable (36%) and the sum of the plasma concentrations of enrofloxacin and ciprofloxacin was maintained at or above 0.1 microg/ml for up to 4 hr. Enrofloxacin appears to be useful for the treatment of goat diseases associated with pathogens sensitive to this drug. |
| Toxicity/Toxicokinetics |
Interactions The objective of the study was to determine the in vitro interaction between enrofloxacin and ciprofloxacin against Escherichia coli and staphylococcal isolates from dogs. The microdilution checkerboard assay was used to determine the interaction of the drugs against 50 E. coli and 50 beta-haemolytic staphylococcal clinical isolates. The checkerboard assay revealed that the activity of enrofloxacin and ciprofloxacin was additive against E. coli and staphylococcal clinical isolates. It was concluded that for bacterial species against which ciprofloxacin is more potent than enrofloxacin, the in vivo transformation of enrofloxacin to ciprofloxacin may enhance the efficacy of enrofloxacin, if additivity of the drugs is confirmed in vivo. |
| References |
[1]. Antibiotic susceptibility profiles of Mycoplasma bovis strains isolated from cattle in Hungary, Central Europe. BMC Vet Res. 2014 Oct 25;10:256. [2]. Superiority of preventive antibiotic treatment compared with standard treatment of poststroke pneumonia in experimental stroke: a bed to bench approach. J Cereb Blood Flow Metab. 2013 Jun;33(6):846-54. [3]. Antiviral activity and inhibition of topoisomerase by ofloxacin, a new quinolone derivative. Antiviral Res. 1987 Oct;8(3):103-13. |
| Additional Infomation |
Enrofloxacin is a quinolinemonocarboxylic acid that is 1,4-dihydroquinoline-3-carboxylic acid substituted by an oxo group at position 4, a fluoro group at position 6, a cyclopropyl group at position 1 and a 4-ethylpiperazin-1-yl group at position 7. It is a veterinary antibacterial agent used for the treatment of pets. It has a role as an antibacterial agent, an antineoplastic agent and an antimicrobial agent. It is a quinolinemonocarboxylic acid, a quinolone, an organofluorine compound, a N-alkylpiperazine, a N-arylpiperazine and a member of cyclopropanes. Enrofloxacin is an antibiotic agent from the fluoroquinolone family produced by the Bayer Corporation. Enrofloxacin is approved by the FDA for its veterinary use. Due to the identification of fluoroquinolone-resistant strains of Campylobacter, in September 2005, the FDA withdrew the approval of enrofloxacin for its use in water to treat flocks of poultry. A fluoroquinolone antibacterial and antimycoplasma agent that is used in veterinary practice. See also: Enrofloxacin; Silver sulfadiazine (component of). |
Solubility Data
| Solubility (In Vitro) |
4-Methylpyridine : ~30 mg/mL H2O : ~1 mg/mL (~2.78 mM) DMSO : 1~10 mg/mL ( 2.78~27.82 mM ) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1 mg/mL (2.78 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1 mg/mL (2.78 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 1 mg/mL (2.78 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 1 mg/mL (2.78 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7825 mL | 13.9125 mL | 27.8249 mL | |
| 5 mM | 0.5565 mL | 2.7825 mL | 5.5650 mL | |
| 10 mM | 0.2782 mL | 1.3912 mL | 2.7825 mL |