Physicochemical Properties
| Molecular Formula | C15H10NO4I4-.NA+ |
| Molecular Weight | 798.850000000001 |
| Exact Mass | 798.669 |
| Elemental Analysis | C, 22.55; H, 1.26; I, 63.54; N, 1.75; Na, 2.88; O, 8.01 |
| CAS # | 137-53-1 |
| Related CAS # | D-Thyroxine;51-49-0 |
| PubChem CID | 23690433 |
| Appearance | Solid powder |
| Boiling Point | 576.3ºC at 760mmHg |
| Flash Point | 302.3ºC |
| Vapour Pressure | 4.02E-14mmHg at 25°C |
| LogP | 3.922 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 25 |
| Complexity | 426 |
| Defined Atom Stereocenter Count | 1 |
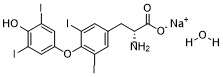
| SMILES | [Na+].O=C(C(CC1C=C(I)C(OC2C=C(I)C(O)=C(I)C=2)=C(I)C=1)N)[O-] |
| InChi Key | YDTFRJLNMPSCFM-UTONKHPSSA-M |
| InChi Code | InChI=1S/C15H11I4NO4.Na/c16-8-4-7(5-9(17)13(8)21)24-14-10(18)1-6(2-11(14)19)3-12(20)15(22)23;/h1-2,4-5,12,21H,3,20H2,(H,22,23);/q;+1/p-1/t12-;/m1./s1 |
| Chemical Name | sodium (R)-2-amino-3-(4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl)propanoate |
| Synonyms | D-Thyroxine sodium salt; 137-53-1; DEXTROTHYROXINE SODIUM; Detyroxin; dynothel; Biotirmone; Choloxin; Debetrol; |
| HS Tariff Code | 2934.99.03.00 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Endogenous Metabolite |
| ln Vitro |
d-Thyroxine (d-T4) is a by-product in the synthesis of l-T4 and is not active in the thyroid.
Dihydroxycoumarin's pharmacological actions are also influenced by D-thyroxine sodium [2]. D-thyroxine is the D-enantiomer of thyroxine. It is a thyroxine and a D-tyrosine derivative. It is an enantiomer of a L-thyroxine. The major hormone derived from the thyroid gland. Thyroxine is synthesized via the iodination of tyrosines (monoiodotyrosine) and the coupling of iodotyrosines (diiodotyrosine) in the thyroglobulin. Thyroxine is released from thyroglobulin by proteolysis and secreted into the blood. Thyroxine is peripherally deiodinated to form triiodothyronine which exerts a broad spectrum of stimulatory effects on cell metabolism. Dextrothyroxine is the dextrorotary isomer of thyroxine, a thyroid hormone with antihyperlipidemic activity. Dextrothyroxine stimulates the formation of low-density lipoprotein (LDL) and increases the catabolism of LDL thereby leading to increased excretion of cholesterol and bile acids via the biliary route. This results in a reduction in serum cholesterol and LDL. |
| ln Vivo | D-thyroxine sodium decreases the metabolism of pentobarbital, pethidine, and dihydroxycoumarin in mice [1]. |
| Enzyme Assay |
Amperometric biosensor for the assay of d-T4[2] Paraffin oil and graphite powder were mixed in a ratio 1:4 (w/w) to form a graphite paste. Hundred microliters from the enzymatic solution (1 mg enzyme ml−1 of 0.1 mol l−1 phosphate buffer, pH 7.0) of l-amino acid oxidase (l-AAOD) (EC 1.4.3.2, Type I: crude dried venom from Crotalus adamanteus) were added to the carbon paste. A plastic tip was filled with the corresponding graphite–paraffin oil paste leaving an empty space of 3–4 mm in the top part filled with carbon paste containing the enzyme. The diameter of the sensor was 3 mm. Electric contact was obtained by inserting a silver wire into the carbon paste. The electrode tip was gently rubbed on fine paper to produce a flat surface. The surface of the electrode was wetted with de-ionized water and then polished with an alumina paper (polished strips 30144-001) before use. The biosensors were stored dry at 4 °C. |
| Animal Protocol | In mice, D-thyroxine inhibits the metabolism of bishydroxycoumarin as well as that of meperidine and pentobarbital; in man, the anticoagulant response to bishydroxycoumarin is increased at doses which do not change the rate of metabolism of the drug. Clofibrate does not affect bishydroxycoumarin metabolism in mice or in man but does increase the anticoagulant response to the drug in man. Norethandrolone also increases the anticoagulant response to bishydroxycoumarin in man without affecting the rate of metabolism of the drug. Since clinically effective doses of these three drugs do not decrease the concentration of vitamin K-dependent clotting factors or affect the absorption, distribution, or metabolism of bishydroxycoumarin in man, it seems likely that they potentiate the pharmacologic effect of bishydroxycoumarin by increasing the affinity of the receptor site for the anticoagulant [1]. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Thyroid is an animal-derived mixture of levothyroxine (T4) and liothyronine (T3), which are normal components of human milk. Limited data on exogenous replacement doses of levothyroxine during breastfeeding indicate no adverse effects in infants. If thyroid is required by the mother, it is not a reason to discontinue breastfeeding. The American Thyroid Association recommends that subclinical and overt hypothyroidism should be treated with levothyroxine in lactating women seeking to breastfeed. Thyroid dosage requirement may be increased in the postpartum period compared to prepregnancy requirements patients with Hashimoto's thyroiditis. ◉ Effects in Breastfed Infants Effects of exogenous thyroid hormone administration to mothers on their infant have not been reported. One case of apparent mitigation of cretinism in hypothyroid infants by breastfeeding has been reported, but the amounts of thyroid hormones in milk are not optimal, and this result has been disputed. The thyroid hormone content of human milk from the mothers of very preterm infants appears not to be sufficient to affect the infants' thyroid status. The amounts of thyroid hormones in milk are apparently not sufficient to interfere with diagnosis of hypothyroidism. In a telephone follow-up study, 5 nursing mothers reported taking levothyroxine (dosage unspecified). The mothers reported no adverse reactions in their infants. One mother with who had undergone a thyroidectomy was taking levothyroxine 100 mcg daily as well as calcium carbonate and calcitriol. Her breastfed infant was reportedly "thriving" at 3 months of age. ◉ Effects on Lactation and Breastmilk Adequate thyroid hormone serum levels are required for normal lactation. Replacing deficient thyroid levels should improve milk production caused by hypothyroidism. Supraphysiologic doses would not be expected to further improve lactation. |
| References |
[1]. John J. Schrogie M.D., et al. II. The effect of D-thyroxine, clofibrate, and norethandrolone. Clinical Pharmacology and Therapeutics, Volume8, Issue1part1 January 1967 Pages 70-77 [2]. Raluca-IoanaStefan, et al. Simultaneous determination of l-thyroxine (l-T4), d-thyroxine (d-T4), and l-triiodothyronine (l-T3) using a sensors/sequential injection analysis system. |
| Additional Infomation |
The dextrorotary isomer of the synthetic THYROXINE. See also: Dextrothyroxine Sodium (annotation moved to). |
Solubility Data
| Solubility (In Vitro) | DMSO: >10 mM |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.2518 mL | 6.2590 mL | 12.5180 mL | |
| 5 mM | 0.2504 mL | 1.2518 mL | 2.5036 mL | |
| 10 mM | 0.1252 mL | 0.6259 mL | 1.2518 mL |