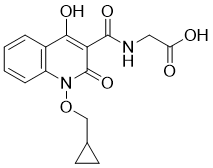
Desidustat is an antianaemic drug candidate under investigation for the treatment of anemia of chronic kidney disease. It acts as an inhibitor of HIF hydroxylase.
Desidustat (Oxemia™) is an orally bioavailable, small molecule, hypoxia-inducible factor-prolyl hydroxylase (HIF-PH) inhibitor developed by Zydus Cadila for the treatment of anaemia associated with chronic kidney disease (CKD), COVID-2019 infections and chemotherapy induced anaemia. Desidustat inhibits prolyl hydroxylase domain enzymes, resulting in the stabilisation of hypoxia-inducible factor which stimulates erythropoietin production and erythropoiesis. In March 2022, desidustat received its first approval in India for the treatment of anaemia in adults with CKD who are either on dialysis or not on dialysis. Desidustat is in clinical development in China for the treatment of anaemia in patients with CKD, in Mexico for the management of COVID-2019 infections and in the USA for the treatment of chemotherapy induced anaemia. This article summarizes the milestones in the development of desidustat leading to this first approval for anaemia associated with CKD. Drugs. 2022 Jul;82(11):1207-1212.Physicochemical Properties
| Molecular Formula | C16H16N2O6 |
| Molecular Weight | 332.308044433594 |
| Exact Mass | 332.1 |
| Elemental Analysis | C, 57.83; H, 4.85; N, 8.43; O, 28.89 |
| CAS # | 1616690-16-4 |
| Related CAS # | 1616690-16-4; |
| PubChem CID | 75593290 |
| Appearance | White to off-white solid powder |
| Density | 1.5±0.1 g/cm3 |
| Index of Refraction | 1.676 |
| LogP | 0.54 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 24 |
| Complexity | 583 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=C(O)CNC(C1=C(O)C2=C(N(OCC3CC3)C1=O)C=CC=C2)=O |
| InChi Key | IKRKQQLJYBAPQT-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C16H16N2O6/c19-12(20)7-17-15(22)13-14(21)10-3-1-2-4-11(10)18(16(13)23)24-8-9-5-6-9/h1-4,9,21H,5-8H2,(H,17,22)(H,19,20) |
| Chemical Name | 2-[[1-(cyclopropylmethoxy)-4-hydroxy-2-oxoquinoline-3-carbonyl]amino]acetic acid |
| Synonyms | Desidustat; 1616690-16-4; ZYAN1; Zyan-1; Desidustat [INN]; Oxemia; ZYAN1 compound; Y962PQA4KS; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | HIF hydroxylase |
| ln Vitro | Performance-enhancing substances and methods have become a serious problem in competitive sports. The hypoxia-inducible factor (HIF) stabilizers can enhance the organism's capacity for molecular oxygen transport and are likely to be abused as performance-enhancing agents in sports. This paper describes the metabolic conversion of the popular hypoxia inducible factor-prolyl hydroxylase (HIF-PH) inhibitors, namely, daprodustat, Desidustat , and vadadustat using equine liver microsomes, determined on a QExactive high-resolution mass spectrometer. During this study, a total of 10 metabolites for daprodustat (all are Phase I), 10 metabolites for Desidustat (five each for Phase I and Phase II), and 15 metabolites for vadadustat (six for Phase I and nine for Phase II) were detected. The important findings of the current research are as follows: (1) All the three HIF-PH inhibitor drug candidates are prone to oxidation, which results in corresponding hydroxylated metabolites; (2) in Desidustat , hydrolysis and dissociation of oxime linkage also observed; (3) the glucuronic acid conjugate (except daprodustat) of the parent drugs as well as the monohydroxylated analogs were observed; (4) sulfonic acid conjugated metabolites were observed only for vadadustat [1]. |
| ln Vivo |
In vivo, desindusat (oral; 10-100 mg/kg) exhibits good effectiveness [1]. Chronic kidney disease (CKD) is associated with activated inflammatory responses. Desidustat, a prolyl hydroxylase (PHD) inhibitor is useful for treatment of anemia associated with CKD, but its effect on the inflammatory and fibrotic changes in CKD is not evaluated. In this study, we investigated the effect of desidustat on the inflammatory and fibrotic changes in preclinical models of acute and chronic kidney injury. Acute kidney injury was induced in male Sprague Dawley rats by ischemia–reperfusion, in which effect of desidustat (15 mg/kg, PO) was estimated. In a separate experiment, male C57 mice were treated with adenine for 14 days to induce CKD. These mice were treated with desidustat (15 mg/kg, PO, alternate day) treatment for 14 days, with adenine continued. Desidustat prevented elevation of serum creatinine, urea, IL-1β, IL-6, and kidney injury molecule-1 (KIM-1), and elevated the erythropoietin levels in rats that were subjected to acute kidney injury. Mice treated with adenine developed CKD and anemia, and desidustat treatment caused improvement in serum creatinine, urea, and also improved hemoglobin and reduced hepatic and serum hepcidin. A significant reduction in IL-1β, IL-6, myeloperoxidase (MPO) and oxidative stress was observed by desidustat treatment. Desidustat treatment also reduced renal fibrosis as observed by histological analysis and hydroxyproline content. Desidustat treatment reduced the renal fibrosis and inflammation along with a reduction in anemia in preclinical models of kidney injury, which may translate to protective effects in CKD patients [1]. Autoimmune hemolytic anemia (AIHA) is a heterogeneous group of diseases mediated by autoantibody directed against RBCs causing hemolysis and anemia. AIHA develops rapidly or over time, depending on the triggering factor. Desidustat is a prolyl hydroxylase inhibitor clinically used for the treatment of chronic kidney disease (CKD)-induced anemia. In this study, we investigated the effect of desidustat in preclinical model of AIHA. We used rat RBC for induction of AIHA in mice. These mice were then treated with desidustat (15 mg/kg, PO, once a day) for eight weeks. Desidustat treatment increased hemoglobin, RBC and hematocrit and decreased WBC and lymphocytes. This treatment suppressed serum LDH, oxidative stress in RBCs, antibody titer and antibody deposition on RBC surface, and increased RBC lifespan. Serum and spleen iron along with spleen mass and oxidative stress were decreased by desidustat. Bone marrow iron was increased and expression of CD71 (cell surface marker for early erythroid progenitor) and TER-119 (cell surface marker for late erythroid progenitor) in bone marrow were found to be elevated by desidustat by treatment. This treatment also suppressed deposition of membrane-bound antibody in late erythroid cells. The treatment showed reduction in total splenic cells, CD71 and TER-119 positive cells in the spleen. Thus, desidustat treatment increased erythropoiesis, early maturation of bone marrow erythroid cells having longer RBC life span due to decrease in the antibody-mediated lysis of RBCs and its progenitors leading to reduced oxidative stress. Thus, desidustat can be a good therapeutic option for treatment of AIHA [2]. |
| Cell Assay | In vitro, Caco2 cell permeability, plasma protein binding, metabolism, cytochrome P450 (CYP) inhibition, and CYP induction were examined [4]. |
| Animal Protocol |
Animal/Disease Models: C57 mice[1] Doses: 10, 30, 50, 100 mg/kg; 20 mg/kg Route of Administration: po (oral gavage); oral administration, one time/day for 7 days. Experimental Results: EPO and Hb levels demonstrated significant rising. Acute kidney injury in SD rats [1] Male SD rats (age 7–8 weeks, and body weight 210–240 g) were anesthetized with ketamine (50 mg/kg, IP) and xylazine (10 mg/kg, IP) and both the renal pedicles were exposed after laparotomy. Bilateral renal pedicles were occluded with hemostasis clamp for 25 min. At the end of bilateral ischemia, the clips were removed to allow reperfusion of blood in kidney. The skin and muscle were closed by surgical suture 3.0. Vehicle in ischemia reperfusion injury (I/R) group (n = 9) or Desidustat (15 mg/kg, PO, n = 9) were administered 30 min prior to and 2 h after to initiation of renal ischemia. In sham (normal) control animals only renal pedicles were exposed after laparotomy. After 24 h of reperfusion, all the animals were bled retro-orbitally under isoflurane anesthesia for collection of blood. Serum samples were analyzed for creatinine, urea, IL-1β, IL-6, erythropoietin, and kidney injury molecule, KIM-1 levels using assay kits mentioned in parentheses. Chronic kidney disease in C57 mice [1] Chronic kidney disease was induced in C57 mice (age 7–8 weeks, and body weight 25–30 g) by adenine supplementation. Animals were randomized based on their body weight into two groups; Adenine group (n = 8), and Desidustat (15 mg/kg, n = 8). All these groups were fed with adenine at 50 mg/kg, by oral route (PO) for 14 days, and for next 14 days they were given Desidustat treatment on alternate day for 14 days, with adenine supplementation continued. A chow-fed control group was separately maintained. On 15th day, the animals were kept in metabolic cages for collection of urine. Next day, animals were bled retro-orbitally under isoflurane anesthesia and serum was separated. Albumin, creatinine, and urea were also measured using Labcare Diagnostics kits. Estimated glomerular filtration rate was calculated as described in literature (Pestel, Krzykalla, & Weckesser, 2007). IL-1β, IL-6 levels using assay kits mentioned in parentheses. Hepcidin in serum and liver were measured using ELISA kit. Lipid peroxidation products, in terms of thiobarbituric reacting substances (TBARS), were measured in kidneys collected immediately after sacrifice, and stored at −70°C until the assay. TBARS was assessed by a spectrophotometric method (Buege, & Aust, 1978). Lipid peroxidation was expressed in terms of malondialdehyde (MDA) equivalents using an extinction coefficient of 1.56 X 105 L / mol per cm and results are expressed as nmol MDA / g tissue. Superoxide dismutase (SOD) activity in kidney was measured spectrophotometrically by the inhibition of pyrogallol auto-oxidation at 420 nm for 10 min (Marklund, & Marklund, 1974). One unit activity was determined as the amount of enzyme that inhibited the oxidation of pyrogallol by 50%. Myeloperoxidase (MPO) was measured in the serum and kidneys (Kim et al., 2012). In addition, kidneys were also processed for the determination of hydroxyproline using Quickzyme hydroxyproline assay kit. Induction of autoimmune hemolytic anemia [2] Autoimmune hemolytic anemia (AIHA) was induced in mice by repeated injections of rat erythrocytes (RBCs). SD rats were bled by retroorbital puncture under isoflurane anesthesia in a tube containing disodium EDTA as an anticoagulant. Plasma was removed and cells were washed three times with phosphate-buffered saline (PBS, pH 7.4) and cells adjusted to a concentration of 1 × 109 cells/ml in PBS. Mice were given injections of approximately 2 × 108 rat RBCs by intraperitoneal route once in a week. After six weeks of initiation of weekly rat RBC injections, mice blood was collected from the tail vein for estimation of complete blood count and were randomized based on RBC, and hemoglobin content. The groups were vehicle control (10 mL/kg), or Desidustat (15 mg/10 mL/kg, once a day, PO) treatment for next eight weeks. Injection of rat RBC was also continued with this treatment for next eight weeks. At the end of treatment, whole blood (for serum and complete blood count), bone marrow, liver and spleen were collected. Lactate dehydrogenase activity was measured in serum samples using a kit in Cobas 6000 instrument. In vivo, pharmacokinetic studies of oral bioavailability in mice, rats, dogs and monkeys, dose linearity, tissue distribution, and excretion in rats were conducted.[4] |
| ADME/Pharmacokinetics |
In Caco-2 cells, the apparent permeability of desidustat was high at low pH and low at neutral pH. The oral bioavailability (%F) of desidustat was 43–100% with a median time to reach peak concentration (Tmax) of about 0.25–1.3 h across species. Desidustat displayed a low mean plasma clearance (CL) of 1.3–4.1 mL/min/kg (approximately 1.8–7.4% of hepatic blood flow), and the mean steady-state volume of distribution (Vss) was 0.2–0.4 L/kg (approximately 30–61% of the total body water). Desidustat showed a dose-dependent increase in exposures over the 15–100 mg/kg dose range. It was rapidly distributed in various tissues, with the highest tissue-to-blood ratio in the liver (1.8) and kidney (1.7). Desidustat showed high plasma protein binding and was metabolically stable in human liver microsomes, hepatocytes, and recombinant CYPs. It did not show significant inhibition of major drug-metabolizing CYP enzymes (IC50 > 300 µM) or the potential to induce CYP1A2 and CYP3A4/5 (up to 100 µM) in HepG2 cells. It may have minimal potential of clinical drug–drug interaction when used in combination with iron supplements or phosphate binders. Desidustat was primarily excreted unchanged in urine (25% of the oral dose) and bile (25% of the oral dose) in rats. The mean elimination half-life of desidustat ranged from 1.0 to 5.3 h and 1.3 to 5.7 h across species after intravenous and oral administration, respectively. [4] Taken together, desidustat is well absorbed orally. It showed a dose-dependent increase in exposure, did not accumulate in tissue, and was eliminated via dual routes. It is metabolically stable, has minimal potential to cause clinical drug–drug interactions (DDIs), and demonstrates discriminable pharmacokinetic properties for the treatment of anemia.[4] |
| References |
[1]. Novel quinolone derivatives. Patent. WO2014102818A1. [2]. Prolyl hydroxylase inhibitor desidustat attenuates autoimmune hemolytic anemia in mice. Int Immunopharmacol. 2024 Dec 5;142(Pt A):113029. [3]. In vitro studies of hypoxia inducible factor-prolyl hydroxylase inhibitors daprodustat, desidustat, and vadadustat for equine doping control. Drug Test Anal. 2022 Feb;14(2):317-348. [4]. Nonclinical Pharmacokinetic Evaluation of Desidustat: a Novel Prolyl Hydroxylase Inhibitor for the Treatment of Anemia. Eur J Drug Metab Pharmacokinet. 2022 Sep;47(5):725-740. |
| Additional Infomation |
Desidustat is a N-acyl-amino acid. Desidustat is under investigation in clinical trial NCT04012957 (Desidustat in the Treatment of Anemia in CKD). Desidustat is an orally bioavailable, hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor (HIF-PHI), with potential anti-anemic and anti-inflammatory activities. Upon administration, desidustat binds to and inhibits HIF-PH, an enzyme responsible for the degradation of transcription factors in the HIF family under normal oxygen conditions. This prevents HIF breakdown and promotes HIF activity. Increased HIF activity leads to an increase in endogenous erythropoietin production, thereby enhancing erythropoiesis. It also reduces the expression of the peptide hormone hepcidin, improves iron availability, and boosts hemoglobin (Hb) levels. HIF regulates the expression of genes in response to reduced oxygen levels, including genes required for erythropoiesis and iron metabolism. In addition, HIF 1-alpha (HIF1A) may play a role in reducing inflammation during acute lung injury (ALI) through HIF-dependent control of glucose metabolism in the alveolar epithelium. Mechanism of Action The small molecule hypoxia-inducible factor, desidustat, inhibits the prolyl hydrozylase and stimulates erythropoiesis. It is currently being investigated against anemia of inflammation and COVID-19. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~10 mg/mL (~30.09 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1 mg/mL (3.01 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1 mg/mL (3.01 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0092 mL | 15.0462 mL | 30.0924 mL | |
| 5 mM | 0.6018 mL | 3.0092 mL | 6.0185 mL | |
| 10 mM | 0.3009 mL | 1.5046 mL | 3.0092 mL |