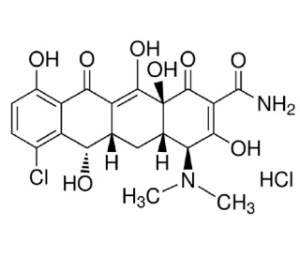
Demeclocycline HCl (also known as demeclocycline hydrochloride; trade names: Detravis; Ledermycin), a protein translation inhibitor and potential calpain inhibitor, is a potent tetracycline antibiotic that inhibits the protein synthesis in bacteria, it is used for the treatment of bacterial infections. Demeclocycline is a semisynthetic tetracycline antibiotic which was derived from a strain of Streptomyces aureofaciens. Demeclocycline binds to bacterial 30S ribosomal subunit and prevents binding of aminoacyl-tRNA to the mRNA-ribosome complex, thereby inhibiting protein synthesis. Demeclocycline also inhibits the effect of vasopressin on the renal tubules, thereby causing diuresis.
Physicochemical Properties
| Molecular Formula | C21H21CLN2O8.HCL | |
| Molecular Weight | 501.31 | |
| Exact Mass | 500.075 | |
| Elemental Analysis | C, 50.31; H, 4.42; Cl, 14.14; N, 5.59; O, 25.53 | |
| CAS # | 64-73-3 | |
| Related CAS # | Demeclocycline;127-33-3 | |
| PubChem CID | 54686764 | |
| Appearance | Solid powder | |
| Boiling Point | 795.9ºC at 760 mmHg | |
| Melting Point | >245°C (dec.) | |
| Flash Point | 435.2ºC | |
| LogP | 1.767 | |
| Hydrogen Bond Donor Count | 7 | |
| Hydrogen Bond Acceptor Count | 9 | |
| Rotatable Bond Count | 2 | |
| Heavy Atom Count | 33 | |
| Complexity | 961 | |
| Defined Atom Stereocenter Count | 5 | |
| SMILES | ClC1C([H])=C([H])C(=C2C(=C3C([C@@]4(C(=C(C(N([H])[H])=O)C([C@]([H])([C@]4([H])C([H])([H])[C@]3([H])[C@@]([H])(C2=1)O[H])N(C([H])([H])[H])C([H])([H])[H])=O)O[H])O[H])=O)O[H])O[H].Cl[H] |
|
| InChi Key | GVSJQNRGSCOSNJ-KBHRXELFSA-N | |
| InChi Code | InChI=1S/C21H21ClN2O8.ClH/c1-24(2)14-7-5-6-10(16(27)12-9(25)4-3-8(22)11(12)15(6)26)18(29)21(7,32)19(30)13(17(14)28)20(23)31;/h3-4,6-7,14-15,25-26,28-29,32H,5H2,1-2H3,(H2,23,31);1H/t6-,7-,14-,15-,21-;/m0./s1 | |
| Chemical Name | (4S,4aS,5aS,6S,12aS)-7-chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide hydrochloride | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Tetracycline |
| References |
[1]. Demeclocycline treatment in the syndrome of inappropriate antidiuretic hormone secretion. Ann Intern Med. 1975 Nov;83(5):654-6. [2]. Demeclocycline-induced nephrogenic diabetes insipidus. In-vivo and in-vitro studies. Ann Intern Med. 1973 Nov;79(5):679-83. [3]. Superiority of demeclocycline over lithium in the treatment of chronic syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med. 1978 Jan 26;298(4):173-7. |
| Additional Infomation |
Demeclocycline Hydrochloride (internal use) can cause developmental toxicity according to state or federal government labeling requirements. Demeclocycline hydrochloride is the hydrochloride salt of demeclocycline. A tetracycline antibiotic, it is used (mainly as the hydrochloride) for the treatment of Lyme disease, acne and bronchitis, as well as for hyponatraemia (low blood sodium concentration) due to the syndrome of inappropriate antidiuretic hormone (SIADH) where fluid restriction alone has been ineffective. It has a role as an antibacterial drug and an aquaretic. It contains a demeclocycline. A TETRACYCLINE analog having a 7-chloro and a 6-methyl. Because it is excreted more slowly than TETRACYCLINE, it maintains effective blood levels for longer periods of time. See also: Demeclocycline (annotation moved to); Demeclocycline Hydrochloride (annotation moved to). |
Solubility Data
| Solubility (In Vitro) |
DMSO : ~100 mg/mL ( ~199.47 mM ) Water : 33.33 ~40 mg/mL(~66.49 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.99 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (4.99 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: font color= ‘FF0000’>10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (4.99 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9948 mL | 9.9739 mL | 19.9477 mL | |
| 5 mM | 0.3990 mL | 1.9948 mL | 3.9895 mL | |
| 10 mM | 0.1995 mL | 0.9974 mL | 1.9948 mL |