Physicochemical Properties
| Molecular Formula | C23H26O11 |
| Molecular Weight | 478.446 |
| Exact Mass | 478.147 |
| CAS # | 53730-90-8 |
| PubChem CID | 101967133 |
| Appearance | Typically exists as solid at room temperature |
| LogP | 0.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 34 |
| Complexity | 1090 |
| Defined Atom Stereocenter Count | 9 |
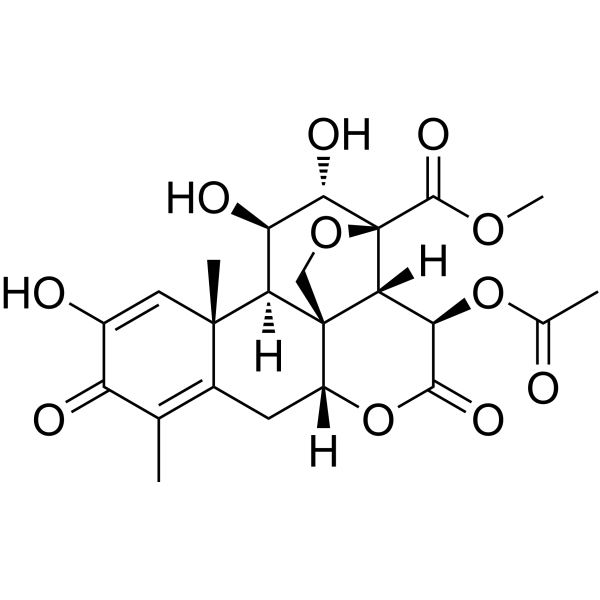
| SMILES | CC1=C2C[C@@H]3[C@@]45CO[C@@]([C@@H]4[C@H](C(=O)O3)OC(=O)C)([C@H]([C@@H]([C@@H]5[C@]2(C=C(C1=O)O)C)O)O)C(=O)OC |
| InChi Key | JXTROYJRNXSSKW-XUVISEOFSA-N |
| InChi Code | InChI=1S/C23H26O11/c1-8-10-5-12-22-7-32-23(20(30)31-4,17(22)15(19(29)34-12)33-9(2)24)18(28)14(27)16(22)21(10,3)6-11(25)13(8)26/h6,12,14-18,25,27-28H,5,7H2,1-4H3/t12-,14-,15-,16-,17-,18+,21+,22-,23+/m1/s1 |
| Chemical Name | methyl (1R,2S,3R,6R,13S,14R,15R,16S,17S)-3-acetyloxy-11,15,16-trihydroxy-9,13-dimethyl-4,10-dioxo-5,18-dioxapentacyclo[12.5.0.01,6.02,17.08,13]nonadeca-8,11-diene-17-carboxylate |
| Synonyms | Dehydrobruceine B; 53730-90-8; methyl (1R,2S,3R,6R,13S,14R,15R,16S,17S)-3-acetyloxy-11,15,16-trihydroxy-9,13-dimethyl-4,10-dioxo-5,18-dioxapentacyclo[12.5.0.01,6.02,17.08,13]nonadeca-8,11-diene-17-carboxylate; methyl (1R,2S,3R,6R,13S,14R,15R,16S,17S)-3-acetyloxy-11,15,16-trihydroxy-9,13-dimethyl-4,10-dioxo-5,18-dioxapentacyclo(12.5.0.01,6.02,17.08,13)nonadeca-8,11-diene-17-carboxylate; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Natural quassinoid; Bax; Bcl-xL; Bcl-2 |
| ln Vitro | In A549 cells, dehydrobruceine B (1 μM; 48 h) and cisplatin (3–18 μM; 48 h) work in concert to produce cytotoxic and apoptotic effects [1]. A549 cells treated with Cisplatin (3 μM, 6 μM; 24 h) produce intracellular ROS in response to Dehydrobruceine B (1 μM) [1]. In A549 cells treated with Cisplatin (3 μM, 6 μM; 24 h), Dehydrobruceine B (3 μM, 6 μM; 24 h) increases depolarization of mitochondrial membrane potential (MMP) and translocation of cytochrome c. In A549 cells, dehydrobruceine B (1 μM; 24 h) amplifies the alterations in pro- and anti-apoptotic protein levels [1]. |
| Enzyme Assay |
Intracellular reactive oxygen species (ROS) determination [1] The intracellular ROS level was determined using 2′,7′-dichlorodihydrofluorescein diacetate as fluorescence probes. After being treated with Dehydrobruceine B (DHB), CDDP and in combination, A549 cells were washed twice with PBS and loaded with 10 μM DCFH-DA for 30 min at 37 °C, then the cells were collected and analyzed by a flow cytometer at an excitation wavelength of 488 nm and an emission wavelength of 530 nm. Analysis of mitochondrial membrane potential (MMP) [1] The MMP was analyzed by 5,5′,6,6′-tetrachloro-1, 1′,3,3′-tetraethyl benzimidazolyl-carbocyanine iodide (JC-1) staining. A549 cells were cultured in 6-well plates, treated with Dehydrobruceine B (DHB), CDDP or in combination, then washed with PBS and incubated in medium containing 2 μM of JC-1 for 30 min at 37 °C. After washing with PBS, the cells were directly observed under a fluorescence microscope. Mitochondrial fractionation [1] Mitochondria were isolated from A549 cells by using Cell Mitochondria Isolation Kit. Briefly, A549 cells were seeded in D-100 dishes and treated with Dehydrobruceine B (DHB), CDDP alone or in combination for 24 h. After being harvested, cells were incubated in 250 μL ice-cold mitochondrial lyses buffer containing 1 mM of PMSF on ice for 15 min, then transferred cell suspension into a pre-chilled Dounce homogenizer, homogenized for 45 strokes on ice. Afterwards, the homogenate was centrifuged at 4 °C, 600 × g for 10 min to remove nuclei and cell debris. The post-nuclear supernatant was centrifuged at 4 °C, 12,000 × g for 15 min to obtain mitochondrial fraction |
| Cell Assay |
Western Blot Analysis[1] Cell Types: A549 cells Tested Concentrations: 1 μM; with or without Cisplatin Incubation Duration: 48 hrs (hours) Experimental Results: Upregulated the protein level of Bax, while downregulated the levels of Bcl-2 and Bcl-xL. Enhanced caspase activation and PARP cleavage. Apoptosis Analysis[1] Cell Types: A549 cells Tested Concentrations: 1 μM; with or without Cisplatin Incubation Duration: 48 hrs (hours) Experimental Results: Inhibited cell viability with 9 μM and 18 μM Cisplatin, respectively. Induced cell apoptosis with 3 μM and 6 μM Cisplatin, respectively. Immunofluorescence[1] Cell Types: A549 cells Tested Concentrations: 1 μM; with or without 3 μM and 6 μM Cisplatin, respectively Incubation Duration: 24 hrs (hours) Experimental Results: Resulted apoptosis-inducing factor (AIF) translocated from cytosol into nucleus dramatically in the co-treatment condition. |
| References |
[1]. Dehydrobruceine B enhances the cisplatin-induced cytotoxicity through regulation of the mitochondrial apoptotic pathway in lung cancer A549 cells. Biomed Pharmacother. 2017 May;89:623-631. |
| Additional Infomation |
Dehydrobruceine B has been reported in Brucea javanica with data available. Dehydrobruceine B (DHB) is a quassinoid isolated from Brucea javanica. We have shown previously that DHB induced apoptosis on two kinds of lung cancer cell lines, A549 and NCI-H292. In the present study, we investigated the interactions of DHB and cisplatin (CDDP) on apoptotic-related cancer cell death. Synergistic effects on cell proliferation and apoptosis were observed when A549 cells were treated with DHB plus CDDP. DHB combined CDDP exposure increased depolarization of mitochondrial membrane potential (MMP) and release of cytochrome c from mitochondria into the cytoplasm. The combination treatment also enhanced protein expression of Bax, reduced the protein levels of Bcl-xL and Bcl-2, and increased the cleavage of caspase-3, caspase-9 and poly (ADP-ribose) polymerase (PARP). These results indicated that DHB sensitized A549 cells to cisplatin by regulating the mitochondrial apoptotic pathway. High constitutive expression of Nrf2 was found in A549 cells, which enhance the resistance of cancer cells to chemotherapeutic agents including cisplatin. DHB reduced the protein levels of Nrf2 and its target genes, which may contribute to the increase of intracellular ROS level, consequently, induced mitochondria apoptosis. These results generated a rationale for further investigation of DHB combined with CDDP as a potential therapeutic strategy in lung cancer. [1] MRP-1 and -2 are major members of MRP family and play important roles in multi-drug resistance (MDR) of cancer cells. It has been clarified that there are AREs found in the promotor region of MRP-1 and -2. As Nrf2 is the main regulator that mediates ARE-driven transcription, so the expression of MRP-1 and -2 may be regulated by Nrf2 pathway. Here, we demonstrated that the protein levels of MRP-1 and -2 were reduced in Dehydrobruceine B (DHB) treated A549 cells. The Nrf2 inhibitory effects also can induce the accumulation of intracellular ROS, which may contribute to the mitochondria dysfunction. Thus, the Nrf2 pathway inhibitory effects may be involved with DHB-induced apoptosis enhancement through decreasing phase II enzymes and transporters expression, increasing intracellular ROS level. As aforementioned, Dehydrobruceine B (DHB) could be used not only as single chemotherapeutic agent, but also could be used as an adjuvant for platinum-based chemotherapy in a sub-toxic dose. However, the poor water solubility limited the bioavailability and in vivo application of DHB. Some drug delivery systems and nanocarriers may contribute to overcome these limitations and thus make it possible for DHB used in vivo. Taken together, this study demonstrated the synergistic effects of DHB and CDDP on cytotoxicity and apoptosis in A549 cells. The data also indicated that DHB augmented the efficacy of CDDP by regulating the mitochondrial apoptotic pathway. Although the inhibition of Nrf2 signaling pathway induced by DHB was revealed, and this might be involved in the potentiation effect to CDDP, however, a detailed mechanistic study about this combination requires additional investigation.[1] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0901 mL | 10.4504 mL | 20.9008 mL | |
| 5 mM | 0.4180 mL | 2.0901 mL | 4.1802 mL | |
| 10 mM | 0.2090 mL | 1.0450 mL | 2.0901 mL |