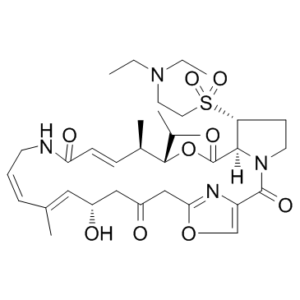
Dalfopristin is a semi-synthetic streptogramin antibiotic analogue of ostreogyrcin A (virginiamycin M, pristinamycin IIA, streptogramin A). Quinupristin/Dalfopristin (Q/D) is a valuable alternative antibiotic to vancomycin for the treatment of multi-drug resistant Enterococcus faecium infections. The combination quinupristin/dalfopristin (marketed under the trade name Synercid) was brought to the market by Rhone-Poulenc Rorer Pharmaceuticals in 1999. Synercid (weight-to-weight ratio of 30% quinupristin to 70% dalfopristin) is used to treat infections by staphylococci and by vancomycin-resistant Enterococcus faecium. Both dalfopristin and quinupristin bind to sites located on the 50S subunit of the ribosome. Initial dalfopristin binding results in a conformational change of the ribosome, allowing for increased binding by quinupristin. A stable drug-ribosome complex is created when the two drugs are used together. This complex inhibits protein synthesis through prevention of peptide-chain formation and blocking the extrusion of newly formed peptide chains. In many cases, this leads to bacterial cell death.
Physicochemical Properties
| Molecular Formula | C34H50N4O9S | |
| Molecular Weight | 690.85 | |
| Exact Mass | 690.33 | |
| Elemental Analysis | C, 59.11; H, 7.30; N, 8.11; O, 20.84; S, 4.64 | |
| CAS # | 112362-50-2 | |
| Related CAS # |
|
|
| PubChem CID | 6323289 | |
| Appearance |
Slightly yellow to yellow powder White solid |
|
| Density | 1.27g/cm3 | |
| Boiling Point | 940.5ºC at 760 mmHg | |
| Melting Point | approximately 150 °C | |
| Flash Point | 522.6ºC | |
| Vapour Pressure | 0mmHg at 25°C | |
| Index of Refraction | 1.575 | |
| LogP | 3.616 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 11 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 48 | |
| Complexity | 1340 | |
| Defined Atom Stereocenter Count | 5 | |
| SMILES | S(C([H])([H])C([H])([H])[N+]([H])(C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])[H])([C@]1([H])C([H])([H])C([H])([H])N2C(C3=C([H])OC(C([H])([H])C(C([H])([H])[C@@]([H])(C([H])=C(C([H])([H])[H])C([H])=C([H])C([H])([H])N([H])C(C([H])=C([H])[C@@]([H])(C([H])([H])[H])[C@@]([H])(C([H])(C([H])([H])[H])C([H])([H])[H])OC(C21[H])=O)=O)O[H])=O)=N3)=O)(=O)=O.S(C([H])([H])[H])(=O)(=O)[O-] |c:48,65,t:55| |
|
| InChi Key | SUYRLXYYZQTJHF-VMBLUXKRSA-N | |
| InChi Code | InChI=1S/C34H50N4O9S/c1-7-37(8-2)16-17-48(44,45)28-13-15-38-31(28)34(43)47-32(22(3)4)24(6)11-12-29(41)35-14-9-10-23(5)18-25(39)19-26(40)20-30-36-27(21-46-30)33(38)42/h9-12,18,21-22,24-25,28,31-32,39H,7-8,13-17,19-20H2,1-6H3,(H,35,41)/b10-9+,12-11+,23-18+/t24-,25-,28-,31-,32-/m1/s1 | |
| Chemical Name | (12Z,32S,33R,6R,7R,8E,13E,15E,17S)-33-((2-(diethylamino)ethyl)sulfonyl)-17-hydroxy-6-isopropyl-7,15-dimethyl-5-oxa-11-aza-1(4,2)-oxazola-3(1,2)-pyrrolidinacycloicosaphane-8,13,15-triene-2,4,10,19-tetraone | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Antibacterial |
| ln Vitro | Streptogramins B (Quinupristin) and Streptogramins A (Dalfopristin) combined at a 30:70 ratio make up Quinupristin/Dalfopristin (Q/D)[1]. Quinupristin/Dalfopristin is a semisynthetic injectable streptogramin that combines two synergistic antibiotic components, Quinupristin (a type B streptogramin) and Dalfopristin (a type A streptogramin), in a 30:70 weight-to-weight ratio. Both components are derived from pristinamycin[2]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Quinupristin and dalfopristin is distributed into milk in rats ... . The pharmacokinetics of quinupristin/dalfopristin have been studied in rats, monkeys and humans following intravenous infusion of radiolabelled and unlabelled drug. In rats and monkeys quinupristin and dalfopristin undergo rapid elimination from the blood and wide tissue distribution. Nevertheless, they do not penetrate the central nervous system or cross the placenta to any significant degree and they do not appear to be subject to significant body retention following cessation of administration. The blood elimination half-life of quinupristin was approximately 0.6 hr in rats and 0.5 hr in monkeys, and that of dalfopristin was approximately 0.6 hr and 0.2 hr, respectively. Both compounds are primarily eliminated through the bile into the faeces; quinupristin is mainly excreted unchanged whereas dalfopristin is extensively metabolized beforehand. The metabolites include the microbiologically active pristinamycin PIIA for dalfopristin and the microbiologically active glutathione- and cysteine-conjugated derivatives for quinupristin. Quinupristin and dalfopristin appear to be handled in a similar manner by humans. Following intravenous administration both compounds are rapidly cleared from the blood with elimination half-lives of approximately 1 hr for quinupristin and 0.4-0.5 hr for dalfopristin. The pharmacokinetic profile of quinupristin is dose-independent and so is that of dalfopristin and RP 12536 when considered together. Extravascular diffusion of quinupristin/dalfopristin has been assessed in human non-inflammatory interstitial fluid. Fecal excretion constitutes the main elimination route for both parent drugs and their metabolites (75 to 77% of dose). Urinary excretion accounts for approximately 15% of the quinupristin and 19% of the dalfopristin dose. Preclinical data in rats have demonstrated that approximately 80% of the dose is excreted in the bile and suggest that in man, biliary excretion is probably the principal route for fecal elimination. Metabolism / Metabolites Converted to an active non-conjugated metabolite by hydrolysis. Quinupristin and dalfopristin are converted to several major active metabolites: 2 conjugated (with glutathione and cysteine) metabolites for quinupristin and one nonconjugated (formed by hydrolysis) metabolite for dalfopristin, which also act synergistically with the complementary parent drug. This conversion occurs in vitro by nonenzymatic reactions independent of cytochrome P-450 (CYP) and glutathione transferase enzymes. Biological Half-Life The elimination half-life is approximately 0.70 hours. The elimination half-life of quinupristin and dalfopristin is approximately 0.85 and 0.70 hours, respectively. The pharmacokinetics of quinupristin/dalfopristin have been studied in rats, monkeys and humans following intravenous infusion of radiolabelled and unlabelled drug. ... The blood elimination half-life of quinupristin was approximately 0.6 hr in rats and 0.5 hr in monkeys, and that of dalfopristin was approximately 0.6 hr and 0.2 hr, respectively. ... Following intravenous administration both compounds are rapidly cleared from the blood with elimination half-lives of approximately 1 hr for quinupristin and 0.4-0.5 hr for dalfopristin. |
| Toxicity/Toxicokinetics |
Protein Binding Moderate Interactions Concomitant administration of Synercid and nifedipine (repeated oral doses) and midazolam (intravenous bolus dose) in healthy volunteers led to elevated plasma concentrations of these drugs. The Cmax increased by 18% and 14% (median values) and the AUC increased by 44% and 33% for nifedipine and midazolam, respectively. In vitro drug interaction studies have demonstrated that Synercid significantly inhibits cytochrome P450 3A4 metabolism of cyclosporin A, midazolam, nifedipine and terfenadine. In addition, 24 subjects given Synercid 7.5 mg/kg q8h for 2 days and 300 mg of cyclosporine on day 3 showed an increase of 63% in the AUC of cyclosporine, an increase of 30% in the Cmax of cyclosporine, a 77% increase in the half life of cyclosporine, and, a decrease of 34% in the clearance of cyclosporine. Therapeutic level monitoring of cyclosporine should be performed when cyclosporine must be used concomitantly with Synercid. A drug interaction between Synercid and digoxin cannot be excluded but is unlikely to occur via CYP3A4 enzyme inhibition. Synercid has shown in vitro activity (MICs of 0.25 ug/mL when tested on two strains) against Eubacterium lentum. Digoxin is metabolized in part by bacteria in the gut and as such, a drug interaction based on Synercid's inhibition of digoxin's gut metabolism (by Eubacterium lentum) may be possible. A case is presented in which a 21-yr-old woman who was receiving 150 mg/day oral cyclosporine after kidney transplantation developed elevated cyclosporine blood levels 2 days after starting treatment with intravenous injections of 20 mg/kg/day quinupristin/dalfopristin. Baseline trough cyclosporine levels ranged from 80 to 105 ng/ml. Two and 3 days after initiation of quinupristin/dalfopristin therapy, trough cyclosporine levels increased to 261 and 291 ng/ml, respectively. The cyclosporine dosage was decreased to 100 mg/day and the blood levels returned to baseline. After discontinuation of quinupristin/dalfopristin, the cyclosporine blood concentration decreased and the dosage was increased to the previous regimen. |
| References |
[1]. Characteristic of Enterococcus faecium clinical isolates with quinupristin/Dalfopristin resistance in China. BMC Microbiol. 2016 Oct 21;16(1):246. [2]. Comparison of antimicrobial agents as therapy for experimental endocarditis: caused by methicillin-resistant Staphylococcus aureus. Tex Heart Inst J. 2010;37(4):400-4. |
| Additional Infomation |
Therapeutic Uses Anti-Bacterial Agents Quinupristin and dalfopristin is used IV in adults for the treatment of serious or life-threatening infections caused by susceptible strains of vancomycin-resistant Enterococcus faecium (VREF), including infections associated with VREF bacteremia. Quinupristin and dalfopristin became commercially available in the US for this indication under the principles and procedures of FDA's accelerated review process that allows approval based on analysis of surrogate markers of response (i.e., clearance of bacteremia), rather than clinical end points such as cure of infection or survival. Controlled clinical studies are underway to confirm the validity of this surrogate marker. /Included in US product labeling/ Quinupristin and dalfopristin is used IV for the treatment of complicated skin and skin structure infections caused by Staphylococcus aureus (methicillin-susceptible strains) or Streptococcus pyogenes (group A beta-hemolytic streptococci). /Included in US product labeling/ The semi-synthetic streptogramin quinupristin/dalfopristin antibiotic exerts potent bactericidal activity against Staphylococcus aureus. /The researchers/ investigated whether, like other bactericidal antibiotics used at subinhibitory concentrations, quinupristin/dalfopristin enhances release of toxins by Gram-positive cocci. The activity of quinupristin/dalfopristin on exotoxin release by S. aureus was investigated by 2D SDS-PAGE combined with MALDI-TOF/MS analysis and by western blotting. /The researchers/ show that quinupristin/dalfopristin at subinhibitory concentrations reduces the release of S. aureus factors that induce tumour necrosis factor secretion in macrophages. Furthermore, quinupristin/dalfopristin but not linezolid attenuated S. aureus-mediated killing of infected host cells. When added to S. aureus cultures at different stages of bacterial growth, quinupristin/dalfopristin reduced in a dose-dependent manner the release of specific virulence factors (e.g. autolysin, protein A, alpha- and beta-haemolysins, lipases). In contrast, other presumably non-toxic exoproteins remained unchanged. The results of the present study suggest that subinhibitory quinupristin/dalfopristin inhibits virulence factor release by S. aureus, which might be especially helpful for the treatment of S. aureus infections, where both bactericidal as well as anti-toxin activity may be advantageous. Drug Warnings Adverse venous effects (e.g., thrombophlebitis, pain) may occur; therefore, flush infusion lines with 5% dextrose injection following completion of peripheral infusions with quinupristin and dalfopristin. Do not flush with sodium chloride injection or heparin solutions because of possible incompatibilities. Recommended measures for moderate-to-severe reactions include increasing the infusion volume, changing infusion sites, or establishing central venous access. Concomitant hydrocortisone or diphenhydramine did not alleviate adverse venous effects during clinical studies. Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible. Because Clostridium difficile-associated diarrhea and colitis has been reported with quinupristin and dalfopristin, ranging in severity from mild to life-threatening, it should be considered in the differential diagnosis of patients who develop diarrhea during or following therapy with the drug. To determine whether myalgias/arthralgias occurring in cancer patients who receive quinupristin/dalfopristin are associated with biliary tract dysfunction, 56 patients with vancomycin-resistant enterococcal infections who were treated with quinupristin/dalfopristin 7.5 mg/kg every 8 hr for a mean duration of 12 days (range 2-52 days) /were studied/. Liver function tests, including a test for alkaline phosphatase, were performed before, during and after the end of therapy. All patients were followed for 1 month after completion of therapy. Thirty-eight (68%) of the 56 patients responded. Myalgias/arthralgias were the leading adverse events occurring in 20 (36%) of the patients. Patients with myalgias/arthralgias had significantly higher levels of alkaline phosphatase (mean 318.7 IU/L) during the mid-term therapy cycle compared with patients without any joint or muscular pain (mean 216.3 IU/L, P = 0.05). In addition, 3/18 (16.6%) patients with myalgias/arthralgias had more than five-fold the normal levels of alkaline phosphatase, which did not occur in any of the other patients who did not develop myalgias/arthralgias (P = 0.04). All myalgias/arthralgias resolved after the discontinuation of quinupristin/dalfopristin. By univariate analysis, other factors associated with myalgias/arthralgias were relapse of hematological malignancy (P = 0.01), receiving tacrolimus within 1 month prior to treatment (P = 0.04) and receiving methotrexate during antimicrobial therapy (P = 0.05). Myalgias/arthralgias occur frequently in cancer patients receiving quinupristin/dalfopristin and may be associated with biliary tract dysfunction, as measured by alkaline phosphatase or other factors that could lead to intra-hepatic cholestasis, such as relapse of haematological malignancy or treatment with tacrolimus or methotrexate. For more Drug Warnings (Complete) data for Dalfopristin (13 total), please visit the HSDB record page. Pharmacodynamics Dalfopristin is a streptogramin antibiotic, derived from pristinamycin IIA. |
Solubility Data
| Solubility (In Vitro) |
DMSO : 100~125 mg/mL ( 144.74~180.94 mM ) Ethanol : ~100 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.01 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (3.01 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (3.01 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.08 mg/mL (3.01 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4475 mL | 7.2375 mL | 14.4749 mL | |
| 5 mM | 0.2895 mL | 1.4475 mL | 2.8950 mL | |
| 10 mM | 0.1447 mL | 0.7237 mL | 1.4475 mL |