Physicochemical Properties
| Molecular Formula | C6H11NO |
| Molecular Weight | 113.16 |
| Exact Mass | 113.084 |
| CAS # | 3317-61-1 |
| PubChem CID | 1774 |
| Appearance | Colorless to light yellow solid if <25°C; liquid if >29°C; Melting Point: 25-29 °C |
| Density | 1.0±0.1 g/cm3 |
| Boiling Point | 274.2±0.0 °C at 760 mmHg |
| Melting Point | 25-29 °C(lit.) |
| Flash Point | 130.7±11.5 °C |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.478 |
| LogP | -1.14 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Heavy Atom Count | 8 |
| Complexity | 127 |
| Defined Atom Stereocenter Count | 0 |
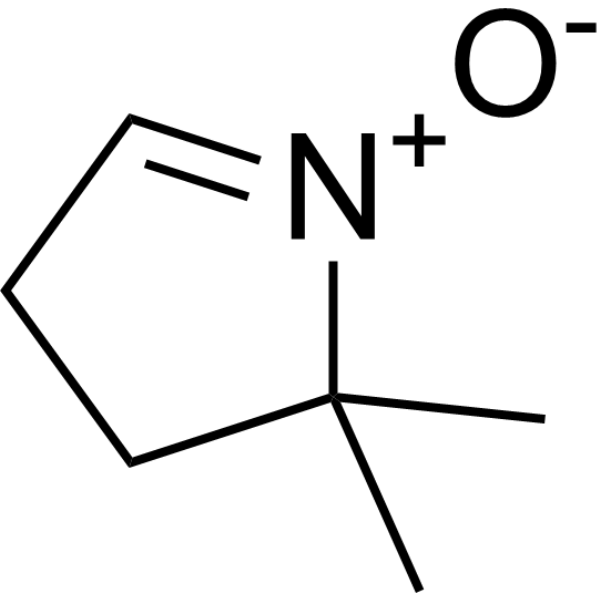
| SMILES | CC1(C)CCC=[N+]1[O-] |
| InChi Key | VCUVETGKTILCLC-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C6H11NO/c1-6(2)4-3-5-7(6)8/h5H,3-4H2,1-2H3 |
| Chemical Name | 2,2-dimethyl-1-oxido-3,4-dihydropyrrol-1-ium |
| Synonyms | 5,5-Dimethyl-1-pyrroline-N-oxide; 3317-61-1; 5,5-Dimethyl-1-pyrroline N-oxide; DMPO; 5,5-Dimethyl-1-pyrroline-N-oxide; 5,5-Dimethyl-1-pyrroline-1-oxide; 2,2-Dimethyl-3,4-dihydro-2H-pyrrole 1-oxide; 2,2-dimethyl-1-oxido-3,4-dihydropyrrol-1-ium; MFCD00005279; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Spin trap agent | ||||||||||||||||||||||
| ln Vitro |
Post-treatment of cells with DMPO attenuated SIN-1-mediated cytotoxicity and ROS generation, restoration of NO levels via increased in eNOS activity and phospho-eNOS levels. Treatment with DMPO alone significantly increased NO levels and induced phosphorylation of eNOS Ser¹¹⁷⁹ via Akt kinase. Transfection studies with wild-type and mutant human eNOS confirmed the dual role of eNOS as a producer of superoxide anion (O₂⁻) with SIN-1 treatment, and a producer of NO in the presence of DMPO [4]. Unlike direct ESR, spin trap methodology depends on the absolute fidelity of the spin trap reaction. Two alternative reactions of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) leading to radical adduct artifacts have been discovered and investigated: inverted spin trapping and the Forrester-Hepburn nucleophilic mechanism. These two alternate pathways to radical adducts are a combination of one-electron oxidation and nucleophilic addition, in either order. In biological systems, serious artifacts have been reported due to the Forrester-Hepburn mechanism, which is initiated by the addition of a nucleophile to DMPO. It has recently been demonstrated that (bi)sulfite (hydrated sulfur dioxide) can react with DMPO via a nonradical, nucleophilic reaction, and it has been further proposed that DMPO/(•)SO(3)(-) formation in biological systems is an artifact and not the result of spin trapping of sulfur trioxide anion radical ((•)SO(3)(-)). The one-electron oxidation of (bi)sulfite catalyzed by horseradish peroxidase (HRP)/hydrogen peroxide (H(2)O(2)) has been reinvestigated by ESR spin trapping with DMPO and oxygen uptake studies to obtain further evidence for the radical reaction mechanism. In the absence of DMPO, the initial rate of (bi)sulfite-dependent oxygen and H(2)O(2) consumption was determined to be half of the initial rate of DMPO/(•)SO(3)(-) radical adduct formation as determined by ESR, demonstrating that, under our experimental conditions, DMPO exclusively forms the radical adduct by trapping the (•)SO(3)(-) [1]. The spin traps substituted with some groups at the 4-position of dimethyl-1-pyrroline N-oxide(DMPO) were compared with DMPO itself regarding their abilities as spin traps and their physical properties. 4,5,5-Trimethyl-1-pyrolline N-oxide (4MDMPO) and 5,5-dimethyl-4-phenyl-1-pyrolline N-oxide (4PDMPO) were synthesized by the Bonnett method, and 5,5-dimethyl-4-hydroxymethyl-1-pyrolline N-oxide (4HMDMPO) was made by a unique method from 2(5H)-furanone. The melting points of 4MDMPO, 4PDMPO and 4HMDMPO were higher than that of DMPO. The magnitude of hydrophilicity was in the order of 4HMDMPO, DMPO, 4MDMPO, and 4PDMPO based on the partition coefficient experiments in a 1-octanol--water system. Several radicals, O2-., HO., .CH3, .CH2OH, .CH(CH3)OH, (CH3)3CO. and H. radicals, were trapped with these DMPO derivatives for comparison with the trapping by DMPO itself. Spin adducts of O2-. with the three DMPO derivatives showed ESR spectra similar to that of DMPO. In spite of the formation of diastereomers arising from spin trapping, the line-width enlargement was very small. The intensities and the decay rates of the spectra of 4MDMPO-O2-, 4PDMPO-O2-, 4HMDMPO-O2- and DMPO-O2- were almost equal. In the trapping of the .OH radical by 4MDMPO, 4PDMPO and 4HMDMPO, the eight-line ESR spectra observed were different from the well-known four-line spectrum of DMPO-OH [2]. |
||||||||||||||||||||||
| ln Vivo | Formalin injected into the left hind paw induced a biphasic nociceptive behaviour. Intraperitoneal injection of DMPO diminished nociceptive behaviors dose-dependently during phase 2 but not phase 1. DMPO (10-100 mg/kg, intraperitoneal injection) has an anti nociceptive effect on formalin induced hyperalgesia in rats [3]. | ||||||||||||||||||||||
| Cell Assay | BAEC were treated with SIN-1, as a source of peroxynitrite anion (ONOO⁻), and then incubated with DMPO. Cytotoxicity following SIN-1 alone and cytoprotection by adding DMPO was assessed by MTT assay. Levels of ROS and NO generation from HEK293 cells transfected with wild-type and mutant eNOS cDNAs, tetrahydrobiopterin bioavailability, eNOS activity, eNOS and Akt kinase phosphorylation were measured [4]. | ||||||||||||||||||||||
| Animal Protocol |
5% formalin was injected in the left hind paw after intraperitoneal injection of saline or various doses of DMPO (10 mg/kg, 30 mg/kg, 100 mg/kg). Number of flinches was measured in a 5 minute interval for 1 hour. Adult male Sprague-Dawley rats (200 |
|