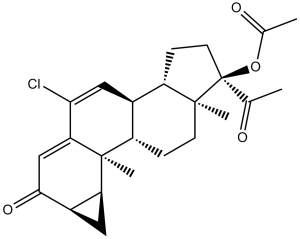
Cyproterone acetate (Cyprone, Androcur, Cyprostat,Cyprohexal, Ciproterona, Cyproteron, Procur, Cyproteronum, Neoproxil, Siterone), an analog of 17-hydroxyprogesterone, is an androgen receptor (AR) antagonist with potential antitumor activity. It inhibits AR with an IC50 of 7.1 nM. Cyproterone acetate works by blocking androgen receptors which prevents androgens from binding to them and suppresses luteinizing hormone, which in turn reduces testosterone levels. It is primarily used to treat prostate cancer, benign prostatic hyperplasia, priapism, hypersexuality and other conditions.
Physicochemical Properties
| Molecular Formula | C24H29CLO4 |
| Molecular Weight | 416.94 |
| Exact Mass | 416.18 |
| Elemental Analysis | C, 69.14; H, 7.01; Cl, 8.50; O, 15.35 |
| CAS # | 427-51-0 |
| Related CAS # | Cyproterone acetate-d3;2376035-90-2 |
| PubChem CID | 9880 |
| Appearance |
Crystals from diisopropyl ether White crystalline powder |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 525.9±50.0 °C at 760 mmHg |
| Melting Point | 200-201ºC |
| Flash Point | 177.6±29.1 °C |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.582 |
| LogP | 3.28 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 29 |
| Complexity | 903 |
| Defined Atom Stereocenter Count | 8 |
| SMILES | C[C@@]12C(C(Cl)=C[C@]3([H])[C@]2([H])C[C@@]4(C)[C@@]3([H])CC[C@]4(OC(C)=O)C(C)=O)=CC([C@H]5[C@@H]1C5)=O |
| InChi Key | UWFYSQMTEOIJJG-FDTZYFLXSA-N |
| InChi Code | InChI=1S/C24H29ClO4/c1-12(26)24(29-13(2)27)8-6-16-14-10-20(25)19-11-21(28)15-9-18(15)23(19,4)17(14)5-7-22(16,24)3/h10-11,14-18H,5-9H2,1-4H3/t14-,15+,16-,17-,18-,22-,23-,24-/m0/s1 |
| Chemical Name | (2aR,3aS,3bS,3cS,5aS,6R,8aS,8bR)-6-acetyl-10-chloro-3b,5a-dimethyl-2-oxo-2,2a,3,3a,3b,3c,4,5,5a,6,7,8,8a,8b-tetradecahydrocyclopenta[a]cyclopropa[g]phenanthren-6-yl acetate |
| Synonyms | Cyprone, Cyprohexal, Ciproterona, Cyproteronum,Androcur, Cyprostat,Cyproteron, Procur, Neoproxil, Siterone.CYPROTERONE ACETATE; 427-51-0; Androcur; Cyproteroneacetate; Cyproterone 17-O-acetate; Cyproteron acetate; Cyproteron-R acetate; Cyprostat; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Androgen receptor (AR) | ||
| ln Vitro | At moderately high doses, cyproterone acetate, a partial agonist, exhibits agonism for the AR with an EC50 of 4.0 μM[1]. By upregulating death receptor 5, cyproterone acetate increases TRAIL-induced androgen-independent prostate cancer cell apoptosis[3]. Pretreatment with cyproterone acetate (0, 1, 10, or 50 μM) for 24 hours, followed by another 24 hours of cadmium exposure, clearly reduced the rat liver epithelial cell line's sensitivity to cadmium[4]. | ||
| ln Vivo | The zona fasciculata and zona retjculans of the adrenal cortex of adult male C57 BL/6J mice injected with 0.08 mg/g of cyproterone acetate had an increase in cellular lipid content[5]. | ||
| Cell Assay |
Background: Virtually all prostate cancer deaths occur due to obtaining the castration-resistant phenotype after prostate cancer cells escaped from apoptosis and/or growth suppression initially induced by androgen receptor blockade. TNF-related apoptosis-inducing ligand (TRAIL) was an attractive cancer therapeutic agent due to its minimal toxicity to normal cells and remarkable apoptotic activity in tumor cells. However, most localized cancers including prostate cancer are resistant to TRAIL-induced apoptosis, thereby creating a therapeutic challenge of inducing TRAIL sensitivity in cancer cells. Herein the effects of cyproterone acetate, an antiandrogen steroid, on the TRAIL-induced apoptosis of androgen receptor-negative prostate cancer cells are reported.[3] Methods: Cell apoptosis was assessed by both annexin V/propidium iodide labeling and poly (ADP-ribose) polymerase cleavage assays. Gene and protein expression changes were determined by quantitative real-time PCR and western blot assays. The effect of cyproterone acetate on gene promoter activity was determined by luciferase reporter assay.[3] Results: Cyproterone acetate but not AR antagonist bicalutamide dramatically increased the susceptibility of androgen receptor-negative human prostate cancer PC-3 and DU145 cells to TRAIL-induced apoptosis but no effects on immortalized human prostate stromal PS30 cells and human embryonic kidney HEK293 cells. Further investigation of the TRAIL-induced apoptosis pathway revealed that cyproterone acetate exerted its effect by selectively increasing death receptor 5 (DR5) mRNA and protein expression. Cyproterone acetate treatment also increased DR5 gene promoter activity, which could be abolished by mutation of a consensus binding domain of transcription factor CCAAT-enhancer-binding protein homologous protein (CHOP) in the DR5 gene promoter. Cyproterone acetate increases CHOP expression in a concentration and time-dependent manner and endoplasmic reticulum stress reducer 4-phenylbutyrate could block cyproterone acetate-induced CHOP and DR5 up-regulation. More importantly, siRNA silencing of CHOP significantly reduced cyproterone acetate-induced DR5 up-regulation and TRAIL sensitivity in prostate cancer cells.[3] Conclusions: Our study shows a novel effect of cyproterone acetate on apoptosis pathways in prostate cancer cells and raises the possibility that a combination of TRAIL with cyproterone acetate could be a promising strategy for treating castration-resistant prostate cancer.[3] |
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Completely absorbed following oral administration. It is excreted approximately 60% in the bile and 33% through the kidneys. Following oral administration, cyproterone acetate is completely absorbed over a wide dose range. The ingestion of two cyproterone acetate 50 mg tablets gives maximum serum levels of about 285 ng/mL at about 3 hours. Thereafter, drug serum levels declined during a time interval of typically 24 to 120 hr, with a terminal half-life of 43.9 +/- 12.8 hr. The total clearance of cyproterone acetate from serum is 3.5 +/- 1.5 mL/min/kg. The absolute bioavailability of cyproterone acetate is almost complete (88% of dose). Some drug is excreted unchanged with bile fluid. Most of the dose is excreted in the form of metabolites at a urinary to biliary ratio of 3:7. Cyproterone acetate is almost exclusively bound to plasma albumin. About 3.5 - 4% of total drug levels are present unbound. Because protein binding is non-specific, changes in SHBG (sex hormone binding globulin) levels do not affect the pharmacokinetics of cyproterone acetate. For more Absorption, Distribution and Excretion (Complete) data for CYPROTERONE ACETATE (6 total), please visit the HSDB record page. Metabolism / Metabolites Primarily hepatic. Cyproterone acetate is metabolized by the CYP3A4 enzyme, forming the active metabolite 15beta-hydroxycyproterone acetate, which retains its antiandrogen activity, but has reduced progestational activity. Cyproterone acetate is metabolized by various pathways, including hydroxylations and conjugations. The main metabolite in human plasma is the 15beta-hydroxy derivative. Biological Half-Life Elimination Following oral or intramuscular administration, the plasma half-life is 38 and 96 hours, respectively. The renal and biliary excretion proceeds with a half-life of 1.9 days. Metabolites from plasma are eliminated at a similar rate (half-life of 1.7 days). Terminal half-life of 43.9 +/- 12.8 hr. |
||
| Toxicity/Toxicokinetics |
Interactions At high therapeutic cyproterone acetate doses of three times 100 mg per day, cyproterone acetate may inhibit CYP2C8. Thiazolidinediones (ie the anti-diabetics pioglitazone and rosiglitazone) are substrates or CYP2C8 (increased blood levels of these anti-diabetics may require dose adjustment). Alcohol appears to reduce the effect of /cyproterone/ which is of no value in chronic alcoholics. The risk of statin-associated myopathy or rhabdomyolysis may be increased when those HMG-CoA inhibitors (statins) which are primarily metabolised by CYP3A4 are co-administered with high cyproterone acetate doses, since they share the same metabolic pathway. Drugs acting on androgen receptors modify opioid transmission in the central nervous system. To investigate a direct interaction, /investigators/ studied whether the binding of [3H]diprenorphine to mouse brain membranes was modified by cyproterone acetate (progesterone derivative with antiandrogen activity), flutamide (non-steroidal antiandrogen), 5alpha-dihydrotestosterone and progesterone. Only cyproterone acetate inhibited [3H]diprenorphine binding (IC50 = (1.62 +/- 0.33) x 10(-6) M) without modifying its association rate. These results suggest that cyproterone acetate binds to opiate receptors independently of its classical androgenic intracellular receptor effect. Non-Human Toxicity Values LD50 Rat intraperitoneal 565 mg/kg LD50 Mouse intraperitoneal 3300 mg/kg |
||
| References |
[1]. Development of androgen- and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol Sci, 2005. 83(1): p. 136-48. [2]. Torri V,. Cyproterone acetate in the therapy of prostate carcinoma. Arch Ital Urol Androl. 2005;77(3):157-163. [3]. Cyproterone acetate enhances TRAIL-induced androgen-independent prostate cancer cell apoptosis via up-regulation of death receptor 5. BMC Cancer. 2017;17(1):179. Published 2017 Mar 7. [4]. Cyproterone acetate induces a cellular tolerance to cadmium in rat liver epithelial cells involving reduced cadmium accumulation. Toxicology. 2001;165(1):13-25. [5]. Migally N. Effect of cyproterone acetate on the structure of the adrenal cortex. Arch Androl. 1979;2(2):109-115. |
||
| Additional Infomation |
Therapeutic Uses Androgen Antagonists; Antineoplastic Agents; Contraceptive Agents, Male; Progestational Hormones, Synthetic /Cyproterone is indicated for the/ control of libido in severe hypersexuality and/or sexual deviation in the adult male. /Cyproterone is indicated for the/ management of patients with prostatic cancer (1) to suppress "flare" with initial LHRH analogue therapy,(2) in long-term palliative treatment where LHRH analogues or surgery are contraindicated, not tolerated, or where oral therapy is preferred, and (3) in the treatment of hot flushes in patients under treatment with LHRH analogues or who have had orchidectomy. Dianette (cyproterone acetate/ethinylestradiol) is recommended for use in women only for the treatment of (a) severe acne, refractory to prolonged oral antibiotic therapy; (b) moderately severe hirsutism. Although Dianette also acts as an oral contraceptive, it should not be used in women solely for contraception, but should be reserved for those women requiring treatment for the androgen-dependent conditions. Drug Warnings Direct hepatic toxicity, including jaundice, hepatitis and hepatic failure, has been observed in patients treated with Cyprostat. At dosages of 100 mg and above cases with fatal outcome have also been reported. Most reported fatal cases were in men with advanced prostatic cancer. Toxicity is dose-related and develops, usually, several months after treatment has begun. Liver function tests should be performed pre-treatment, regularly during treatment and whenever any symptoms or signs suggestive of hepatotoxicity occur. If hepatotoxicity is confirmed, Cyprostat should be withdrawn, unless the hepatotoxicity can be explained by another cause, eg metastatic disease, in which case Cyprostat should be continued only if the perceived benefit outweighs the risk. In very rare cases benign and malignant liver tumors, which may lead to life-threatening intra-abdominal hemorrhage, have been observed after the use of Cyprostat. If severe upper abdominal complaints, liver enlargement or signs of intra-abdominal hemorrhage occur, a liver tumor should be considered in the differential diagnosis. The occurrence of thromboembolic events has been reported in patients using Cyprostat, although a causal relationship has not been established. Patients with previous arterial or venous thrombotic/thromboembolic events (eg deep vein thrombosis, pulmonary embolism, myocardial infarction), with a history of cerebrovascular accidents or with advanced malignancies are at increased risk of further thromboembolic events, and may be at risk of recurrence of the disease during Cyprostat therapy. In patients with a history of thromboembolic processes or suffering from sickle-cell anemia or severe diabetes with vascular changes, the risk: benefit ratio must be considered carefully in each individual case before Cyprostat is prescribed. For more Drug Warnings (Complete) data for CYPROTERONE ACETATE (17 total), please visit the HSDB record page. Pharmacodynamics Cyproterone is an antiandrogen. It suppresses the actions of testosterone (and its metabolite dihydrotestosterone) on tissues. It acts by blocking androgen receptors which prevents androgens from binding to them and suppresses luteinizing hormone (which in turn reduces testosterone levels). |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.20 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.20 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (6.20 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 2 mg/mL (4.96 mM) in 10% EtOH + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear EtOH stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 5: ≥ 2 mg/mL (4.96 mM) (saturation unknown) in 10% EtOH + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear EtOH stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 6: ≥ 2 mg/mL (4.96 mM) (saturation unknown) in 10% EtOH + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.0 mg/mL clear EtOH stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 7: 1%DMSO+30% polyethylene glycol+1%Tween 80: 20 mg/mL Solubility in Formulation 8: 12.5 mg/mL (31.02 mM) in Cremophor EL (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3984 mL | 11.9921 mL | 23.9843 mL | |
| 5 mM | 0.4797 mL | 2.3984 mL | 4.7969 mL | |
| 10 mM | 0.2398 mL | 1.1992 mL | 2.3984 mL |