Physicochemical Properties
| Molecular Formula | C18H20O6 |
| Molecular Weight | 332.3478 |
| Exact Mass | 332.125 |
| CAS # | 109971-63-3 |
| Related CAS # | 109971-63-3 |
| PubChem CID | 5458993 |
| Appearance | White to off-white solid |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 528.4±50.0 °C at 760 mmHg |
| Flash Point | 273.3±30.1 °C |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.627 |
| LogP | 3.84 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 24 |
| Complexity | 387 |
| Defined Atom Stereocenter Count | 0 |
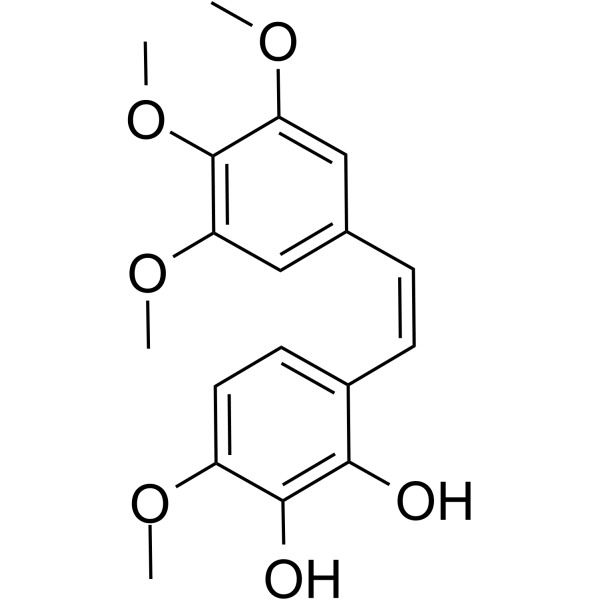
| SMILES | COC1C=CC(/C=C/C2C=C(OC)C(OC)=C(OC)C=2)=C(O)C=1O |
| InChi Key | YUSYSJSHVJULID-WAYWQWQTSA-N |
| InChi Code | InChI=1S/C18H20O6/c1-21-13-8-7-12(16(19)17(13)20)6-5-11-9-14(22-2)18(24-4)15(10-11)23-3/h5-10,19-20H,1-4H3/b6-5- |
| Chemical Name | 3-methoxy-6-[(Z)-2-(3,4,5-trimethoxyphenyl)ethenyl]benzene-1,2-diol |
| Synonyms | Combretastatin A-1 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Microtubule/Tubulin |
| ln Vitro |
Combretastatin A-1 (72 h) suppresses the growth of a number of tumor cell lines in vitro, such as HepG2, SMMC-7721, Hepa 1-6, LM-3, Bel-7402, Huh7, BGC-803, MDA-MB-231, MCF-7, A375, NCI-1975, CT-26, HT-29, and A549 cells (IC50=9.2, 12.8, 32.9, 33.8, 38.4, 728.2, 12.2, 17.6, 46.0, 61.0, 256.3, 1075.0, 2082.0, 2247.0 nM, respectively)[2]. Combretastatin A-1 (1-10 nM; 24 h) causes apoptosis in HepG2 cells by eliminating GSK-3β inhibition and inducing AKT inactivation through microtubule depolymerization[2]. Combretastatin A-1 (1-50 nM; 6 h) reduces the HepG2 cells' mitochondrial membrane potential (MMP). ROS accumulation in HepG2 cells is dose-dependent when using Combretastatin A-1[2]. |
| ln Vivo |
Combretastatin A-1 (1-4 mg/kg; i.v. every other day for 4 weeks) dramatically reduces the tumor volume in HepG2 subcutaneous xenograft model[2]. Combretastatin A-1 (2 mg/kg; every other day for 21 days) exhibits increased apoptosis in orthotopic hepatocellular carcinoma mouse model[2]. |
| Cell Assay |
Cell Line: HepG2 cells Concentration: 1, 5, 10 nM Incubation Time: 24 hours Result: Significantly decreased Mcl-1 expression, but the Bcl-2 level was unchanged. Reduced p-GSK 3β (Ser9) without altering total GSK-3β protein levels, indicating an activation of GSK-3β. Reduced AKT phosphorylation on Ser473 without an obvious change in the total AKT protein levels. |
| Animal Protocol |
Male athymic BALB/c nu/nu mice (16-18 g; 4-6 weeks old) were inoculated with HepG2 cells[2] 1, 2, 4 mg/kg I.v. every other day for 4 weeks |
| References |
[1]. Isolation, structure, and synthesis of combretastatins A-1 and B-1, potent new inhibitors of microtubule assembly, derived from Combretum caffrum. J Nat Prod. Jan-Feb 1987;50(1):119-31. [2]. Combretastatin A-1 phosphate, a microtubule inhibitor, acts on both hepatocellular carcinoma cells and tumor-associated macrophages by inhibiting the Wnt/β-catenin pathway. Cancer Lett. 2016 Sep 28;380(1):134-43. [3]. Anti-tumor and anti-vascular effects of the novel tubulin-binding agent combretastatin A-1 phosphate. Anticancer Res. Nov-Dec 2002;22(6C):3933-40. |
| Additional Infomation |
Combretastatin A-1 has been reported in Combretum caffrum and Combretum kraussii with data available. Combretastatin A-1 is a stilbenoid originally isolated from the plant Combretum caffrum, with vascular-disrupting and antineoplastic activities. Upon administration, combretastatin A1 (CA1) promotes rapid microtubule depolymerization; endothelial cell mitotic arrest and apoptosis; destruction of the tumor vasculature; disruption of tumor blood flow; and tumor cell necrosis. In addition, orthoquinone intermediates, metabolized from combretastatin A1 by oxidative enzymes found to be elevated in some tumor types, may bind to tumor cell thiol-specific antioxidant proteins and DNA, and stimulate oxidative stress by enhancing superoxide/hydrogen peroxide production. |
Solubility Data
| Solubility (In Vitro) | DMSO: ≥ 100 mg/mL (~300.9 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (6.26 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (6.26 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (6.26 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0089 mL | 15.0444 mL | 30.0888 mL | |
| 5 mM | 0.6018 mL | 3.0089 mL | 6.0178 mL | |
| 10 mM | 0.3009 mL | 1.5044 mL | 3.0089 mL |