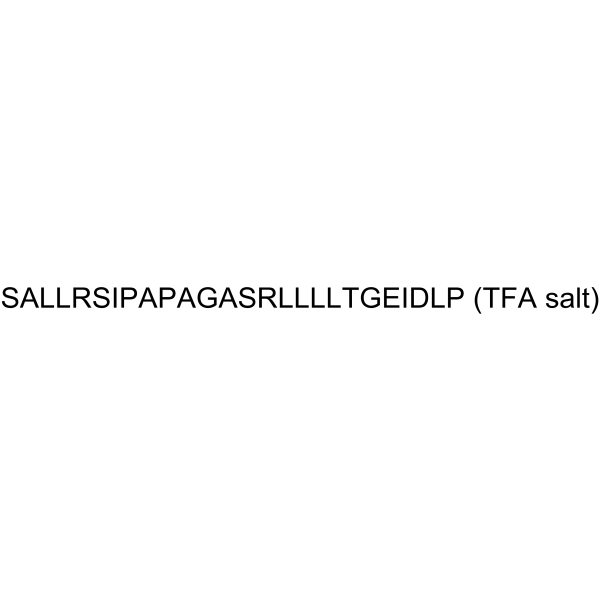Physicochemical Properties
| Molecular Formula | C121H207F3N32O37 |
| Molecular Weight | 2759.13 |
| Exact Mass | 2760.544 |
| CAS # | 2803948-60-7 |
| Related CAS # | 867021-83-8 |
| PubChem CID | 170907493 |
| Appearance | White to off-white solid powder |
| Hydrogen Bond Donor Count | 38 |
| Hydrogen Bond Acceptor Count | 44 |
| Rotatable Bond Count | 86 |
| Heavy Atom Count | 193 |
| Complexity | 5990 |
| Defined Atom Stereocenter Count | 27 |
| SMILES | C(N1CCC[C@H]1C(=O)N[C@@H](C)C(N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@]([H])([C@H](O)C)C(=O)NCC(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(C)C)C(N1CCC[C@H]1C(=O)O)=O)=O)(=O)[C@H]([C@@H](C)CC)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CO.C(F)(F)(F)C(=O)O |
| InChi Key | LVLDZLQHAOAKIR-MZNINCGGSA-N |
| InChi Code | InChI=1S/C119H208N32O35.C2HF3O2/c1-24-63(17)91(113(181)142-80(50-90(160)161)106(174)143-81(49-62(15)16)115(183)151-42-30-35-86(151)117(185)186)146-100(168)73(36-37-89(158)159)133-88(157)52-128-112(180)93(69(23)155)148-107(175)79(48-61(13)14)141-105(173)78(47-60(11)12)140-104(172)77(46-59(9)10)139-103(171)76(45-58(7)8)137-98(166)71(31-26-38-125-118(121)122)135-108(176)82(54-153)144-95(163)66(20)129-87(156)51-127-94(162)65(19)131-110(178)84-33-28-40-149(84)114(182)68(22)132-111(179)85-34-29-41-150(85)116(184)92(64(18)25-2)147-109(177)83(55-154)145-99(167)72(32-27-39-126-119(123)124)134-101(169)75(44-57(5)6)138-102(170)74(43-56(3)4)136-96(164)67(21)130-97(165)70(120)53-152;3-2(4,5)1(6)7/h56-86,91-93,111,132,152-155,179H,24-55,120H2,1-23H3,(H,127,162)(H,128,180)(H,129,156)(H,130,165)(H,131,178)(H,133,157)(H,134,169)(H,135,176)(H,136,164)(H,137,166)(H,138,170)(H,139,171)(H,140,172)(H,141,173)(H,142,181)(H,143,174)(H,144,163)(H,145,167)(H,146,168)(H,147,177)(H,148,175)(H,158,159)(H,160,161)(H,185,186)(H4,121,122,125)(H4,123,124,126);(H,6,7)/t63-,64-,65-,66-,67-,68-,69+,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,91-,92-,93-,111?;/m0./s1 |
| Chemical Name | (2S)-1-[(2S)-2-[[(2S)-2-[[(2S,3S)-2-[[(2S)-2-[[2-[[(2S,3R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[[(2S)-1-[(2S,3S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-hydroxypropanoyl]amino]propanoyl]amino]-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]-5-carbamimidamidopentanoyl]amino]-3-hydroxypropanoyl]amino]-3-methylpentanoyl]pyrrolidin-2-yl]-hydroxymethyl]amino]propanoyl]pyrrolidine-2-carbonyl]amino]propanoyl]amino]acetyl]amino]propanoyl]amino]-3-hydroxypropanoyl]amino]-5-carbamimidamidopentanoyl]amino]-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]-3-hydroxybutanoyl]amino]acetyl]amino]-4-carboxybutanoyl]amino]-3-methylpentanoyl]amino]-3-carboxypropanoyl]amino]-4-methylpentanoyl]pyrrolidine-2-carboxylic acid;2,2,2-trifluoroacetic acid |
| Synonyms | Colivelin |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | STAT3 Amyloid-β |
| ln Vitro | At a concentration of 100 fm, colavelin totally inhibits mortality caused by overexpressed FAD-caused genes and Aβ1-43, while maintaining its neuroprotective effects at or above 1 nm[1]. Colivelin-induced neuroprotection is mediated by two neuroprotective pathways: signal transducer and activator of transcription 3 (STT3), which is initiated by HN, and Ca2+/calmodulin-dependent protein kinase IV, which is mediated by ADNF[1]. In HT22 cells treated with rmMFG -E8, collagen reverses the expressions of caspase-3, Bax, and Bcl-2 in the co-cultured cells under OGD conditions[4]. In BV-2 cells, ivelin (50 µg/mL, 4 hours) dramatically raises the levels of the protein p-STAT3[4]. |
| ln Vivo | Colivelin (intracerebroventricular administration; 10 pmol/3 μl; 3 weeks) antagonizes neuronal loss in the CA1 region of the hippocampus by injecting Aβ1-42 into the hippocampal region, and it suppresses impairment in spatial working-induced memory induced by repetitive intracerebroventricular injection of Aβ25-35 or Aβ1-42[1]. Colivelin suppresses memory impairment caused by 3-quinuclidinyl benzilate and restricts functional memory deficit when administered intraperitoneally at 1.4, 7, or 35 nM/0.21 mL on the Y-maze test day[1]. (intraperitoneal injection; 1 mg/kg; 14 days) over time, as measured by the mNSS, rotarod, and corner turning tests, improves motor and cognitive function. Additionally, it improves neurological deficits following MCAO and decreases lesion volume[1]. |
| Cell Assay |
Western Blot Analysis[4] Cell Types: BV-2 cells. Tested Concentrations: 50 µg/mL. Incubation Duration: 4 hrs (hours). Experimental Results: Increased p-STAT3 levels. Cell Viability Assay[5] Cell Types: KYSE70 and TE8 cells. Tested Concentrations: 0.5 μM. Incubation Duration: 1 hour (followed by CYT-Rx20 treatment) Experimental Results: Dramatically suppressed the viability in KYSE70 and TE8 cells. |
| Animal Protocol |
Animal/Disease Models: CD-1 mice[1] Doses: 10 pmol/3 μl Route of Administration: Intracerebroventricular administration Experimental Results: Completely suppressed Aβ 25-35-mediated impairment in spatial working memory and increased the number of immunoreactive neurons. Animal/Disease Models: C57 mice[1] Doses: 1.4, 7, or 35 nM/0.21mL Route of Administration: intraperitoneal (ip)administration Experimental Results: Protected against cholinotoxin-induced amnesia in mice. Animal/Disease Models: Male C57BL/ 6 mice[3] Doses: 1 mg/kg Route of Administration: intraperitoneal (ip)administration Experimental Results: Protected against ischemic brain injury, and improves neurological outcomes. |
| References |
[1]. Development of a femtomolar-acting humanin derivative named colivelin by attaching activity-dependent neurotrophic factor to its N terminus: characterization of colivelin-mediated neuroprotection against Alzheimer's disease-relevant insults in vitro and in vivo. J Neurosci. 2005 Nov 2;25(44):10252-61. [2]. Colivelin Rescues Ischemic Neuron and Axons Involving JAK/STAT3 Signaling Pathway.Neuroscience. 2019 Sep 15;416:198-206. [3]. Upregulation of HSP72 attenuates tendon adhesion by regulating fibroblast proliferation and collagen production via blockade of the STAT3 signaling pathway.Cell Signal. 2020 Mar 18:109606. [4]. MFG-E8 alleviates oxygen-glucose deprivation-induced neuronal cell apoptosis by STAT3 regulating the selective polarization of microglia. Int J Neurosci. 2020 Mar 12:1-10. [5]. The Synthetic β-Nitrostyrene Derivative CYT-Rx20 Inhibits Esophageal Tumor Growth and Metastasis via PI3K/AKT and STAT3 Pathways. PLoS One. 2016 Nov 22;11(11):e0166453. |
Solubility Data
| Solubility (In Vitro) | H2O : 50 mg/mL (18.12 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 6.25 mg/mL (2.27 mM) in Water with 5% sefsol and 20% isopropanol (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.3624 mL | 1.8122 mL | 3.6243 mL | |
| 5 mM | 0.0725 mL | 0.3624 mL | 0.7249 mL | |
| 10 mM | 0.0362 mL | 0.1812 mL | 0.3624 mL |