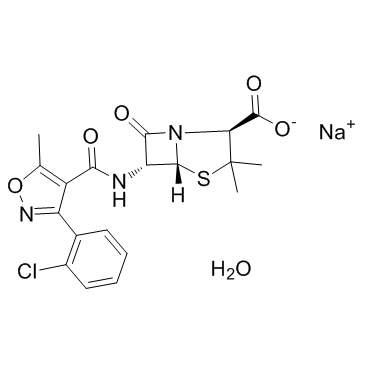
Physicochemical Properties
| Molecular Formula | C19H17CLN3NAO5S |
| Molecular Weight | 457.8632 |
| Exact Mass | 457.047 |
| Elemental Analysis | C, 49.84; H, 3.74; Cl, 7.74; N, 9.18; Na, 5.02; O, 17.47; S, 7.00 |
| CAS # | 7081-44-9 |
| Related CAS # | Cloxacillin sodium;642-78-4;Cloxacillin;61-72-3 |
| PubChem CID | 23675320 |
| Appearance | White to light yellow solid powder. |
| Boiling Point | 689.7ºC at 760 mmHg |
| Flash Point | 370.9ºC |
| LogP | 0.992 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 30 |
| Complexity | 728 |
| Defined Atom Stereocenter Count | 3 |
| SMILES | ClC1=C([H])C([H])=C([H])C([H])=C1C1C(=C(C([H])([H])[H])ON=1)C(N([H])[C@]1([H])C(N2[C@@]([H])(C(=O)[O-])C(C([H])([H])[H])(C([H])([H])[H])S[C@@]21[H])=O)=O.[Na+] |
| InChi Key | InChI=1S/C19H18ClN3O5S.Na/c1-8-11(12(22-28-8)9-6-4-5-7-10(9)20)15(24)21-13-16(25)23-14(18(26)27)19(2,3)29-17(13)23;/h4-7,13-14,17H,1-3H3,(H,21,24)(H,26,27);/q;+1/p-1/t13-,14+,17-;/m1./s1 |
| InChi Code | InChI=1S/C19H18ClN3O5S.Na/c1-8-11(12(22-28-8)9-6-4-5-7-10(9)20)15(24)21-13-16(25)23-14(18(26)27)19(2,3)29-17(13)23;/h4-7,13-14,17H,1-3H3,(H,21,24)(H,26,27);/q;+1/p-1/t13-,14+,17-;/m1./s1 |
| Chemical Name | sodium (2S,5R,6R)-6-(3-(2-chlorophenyl)-5-methylisoxazole-4-carboxamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate |
| Synonyms | Cloxacilina; Cloxacilline; Cloxacillinum; Syntarpen; Tegopen, Cloxacillin sodium. |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: (1). This product requires protection from light (avoid light exposure) during transportation and storage.(2). Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Tetracycline | |
| ln Vitro |
For S. aureus 8325-4 and DU1090, cloxacillin sodium monohydrate (0–2048 µg/mL; 20–24 h) exhibits good antibacterial activity with MIC values of 0.125 µg/mL[1]. The hemolytic activity of Hlα is inhibited in vitro by cloxacillin sodium monohydrate (0.015625 μg/mL; 6 h). This inhibition is enhanced when combined with TZ and TZ. Additionally, cloxacillin sodium monohydrate suppresses the inflammatory response by preventing the activation of MAPKs, NF-кB, and NLRP3-related proteins[1]. |
|
| ln Vivo |
|
|
| Cell Assay |
Cell Line: S. aureus 8325-4, S. aureus DU1090 (an Hlα-deleted strain) Concentration: 0-2048 µg/mL Incubation Time: 20-24 h Result: inhibited DU1090 and S. aureus 8325-4 with MIC values of 0.125 µg/mL. |
|
| Animal Protocol |
Animal Model: Eight weeks old; systemic S. aureus-induced arthritis model; female wildtype C57BL/6 mice) Dosage: 7.5 mg/per (combines with 25 µg/per anti-IL-15 antibodies) Administration: intraperitoneal injection; started on day 3 (following bacterial inoculation) and continued every day until day 6. Result: demonstrated effects on bone erosions and severe synovitis when paired with anti-IL-15 antibodies. |
|
| References |
[1]. The combination of cloxacillin, thioridazine and tetracycline protects mice against Staphylococcus aureus peritonitis by inhibiting α-Hemolysin-induced MAPK/NF-κB/NLRP3 activation. Int J Biol Macromol. 2022 Feb 15;198:1-10. [2]. Antibiotics with Interleukin-15 Inhibition Reduce Joint Inflammation and Bone Erosions but Not Cartilage Destruction in Staphylococcus aureus-Induced Arthritis. Infect Immun. 2018 Apr 23;86(5):e00960-17. [3]. Group 1 beta-lactamases of Aeromonas caviae and their resistance to beta-lactam antibiotics. Can J Microbiol. 2003 Mar;49(3):207-15. |
|
| Additional Infomation |
Cloxacillin sodium is an organic sodium salt. It contains a cloxacillin(1-). Cloxacillin Sodium is the sodium salt of cloxaclliin, a semisynthetic beta-lactamase resistant penicillin antibiotic with antibacterial activity. Cloxacillin binds to and inactivates penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall, thereby preventing the cross-linkage of peptidoglycans, which are critical components of the bacterial cell wall. This leads to an interruption of the bacterial cell wall and causes bacterial cell lysis. Cloxacillin Sodium Anhydrous is the anhydrous form of the sodium salt of cloxacillin, a semisynthetic beta-lactamase resistant penicillin antibiotic with antibacterial activity. Cloxacillin binds to and inactivates penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall, thereby preventing the cross-linkage of peptidoglycans, which are critical components of the bacterial cell wall. This leads to an interruption of the bacterial cell wall and causes bacterial cell lysis. A semi-synthetic antibiotic that is a chlorinated derivative of OXACILLIN. See also: Cloxacillin (has active moiety). |
Solubility Data
| Solubility (In Vitro) |
DMSO : 55~100 mg/mL (115.57~210.14 mM ) Ethanol : ~25 mg/mL H2O : ~50 mg/mL (~105.07 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.25 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.25 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.25 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (5.25 mM) Solubility in Formulation 5: 100 mg/mL (210.14 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1841 mL | 10.9204 mL | 21.8407 mL | |
| 5 mM | 0.4368 mL | 2.1841 mL | 4.3681 mL | |
| 10 mM | 0.2184 mL | 1.0920 mL | 2.1841 mL |