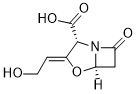
Physicochemical Properties
| Exact Mass | 199.048 |
| CAS # | 58001-44-8 |
| Related CAS # | Potassium clavulanate cellulose;Clavulanate lithium;61177-44-4;Clavulanate potassium;61177-45-5 |
| PubChem CID | 5280980 |
| Appearance | Off-white to light yellow solid powder |
| Density | 1.7±0.1 g/cm3 |
| Boiling Point | 545.8±50.0 °C at 760 mmHg |
| Melting Point |
117.5-118 117.5 - 118 °C |
| Flash Point | 283.9±30.1 °C |
| Vapour Pressure | 0.0±3.3 mmHg at 25°C |
| Index of Refraction | 1.644 |
| LogP | -1.98 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 14 |
| Complexity | 324 |
| Defined Atom Stereocenter Count | 2 |
| SMILES | C(=C/1\[C@H](C(=O)O)N2C(=O)C[C@H]2O1)/CO |
| InChi Key | HZZVJAQRINQKSD-PBFISZAISA-N |
| InChi Code | InChI=1S/C8H9NO5/c10-2-1-4-7(8(12)13)9-5(11)3-6(9)14-4/h1,6-7,10H,2-3H2,(H,12,13)/b4-1-/t6-,7-/m1/s1 |
| Chemical Name | (2R,3Z,5R)-3-(2-hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| Synonyms | Clavulanate; Acide clavulanique; Acido clavulanico; Clavulansaeure; Antibiotic MM 14151; acidum clavulanicum; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: (1). This product requires protection from light (avoid light exposure) during transportation and storage.(2). Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam |
| ln Vitro |
Clavulanic acid and ampicillin exhibit synergistic antibacterial activity (against β-lactamase-producing organisms)[2]. Ab11 and Ab51 strains are inhibited by clavulanic acid at MICs of 2–8 μg/mL[3]. |
| ln Vivo |
An A. baumannii-infected C57BL/6 mouse pneumonia model's lung bacterial load is reduced by clavulanic acid (13 mg/kg, i.p.)[3].
Clavulanic acid (13 mg/kg, i.p.) in the pneumonia model of Ab51-infected C57BL/6 mice exhibits a t1/2 of 6.69 h and an AUC of 4.03 mg·h/L[3]. Rat models of paw edema induced by carrageenan (HY-125474) exhibit anti-inflammatory effects when treated with clavulanic acid (100–300 mg/kg, i.p.)[4]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Clavulanic acid, when taken orally, is well absorbed in the gastrointestinal tract. After administration of radiolabeled clavulanic acid to four human subjects, a minimum of 73% absorption and the average absolute bioavailability was calculated at 64%. The mean Cmax in a group of 8 healthy research volunteers was 2.098 ± 0.441 micrograms/ml in a pharmacokinetic study. The same study reported a mean Tmax of 1.042 ± 0.80 hours. Tmax is reported to be 40-120 minutes according to another pharmacokinetic study. About 40 to 65% of the clavulanic acid is excreted as unchanged drug in urine during the first 6 hours following ingestion. The metabolites of clavulanic acid are found to be excreted in the urine and feces and as carbon dioxide in expired air. Clavulanate is cleared by both renal and non-renal processes. About 17% of radiolabeled dose of clavulanic acid was found to be exhaled in expired air and 8% of a dose was found to be excreted in the feces. A study in 4 healthy volunteers administered a radiolabeled dose of clavulanic acid determined a volume of distribution of 12L.Clavulanic acid is distributed to various tissues and interstitial fluid. Clinically significant concentrations have been measured in the gallbladder, abdomen, skin, fat, and muscle tissues. Bile, pus, synovial and peritoneal fluids are also found to have therapeutic concentrations of clavulanic acid. Studies of animals have demonstrated that clavulanic crosses the placenta. The clearance of clavulanic acid in a pharmacokinetic study of 4 healthy volunteers administered a radiolabeled dose of clavulanic acid was 0.21 l/min. Another resource indicates the average clearance of clavulanic acid is 12.20 liters/h/70 kg. Dose adjustments may be required in patients with renal failure. Metabolism / Metabolites Clavulanic acid is heavily metabolized to form the metabolites 2,5-dihydro-4-(2- hydroxyethyl)-5-oxo-1H-pyrrole-3-carboxylic acid and 1-amino-4-hydroxy-butan-2-one. The first metabolite was found to account for 15.6% of the dose while the second metabolite was reported to account for 8.8% of the dose in one pharmacokinetic study. Biological Half-Life The half-life of clavulanic acid is reported to be similar to amoxicillin, and last 45-90 minutes. A study of radiolabeled clavulanic acid administered to 4 healthy volunteers determined a half-life of 0.8 h. |
| Toxicity/Toxicokinetics |
Protein Binding The plasma protein binding of amoxicillin is about 25%. |
| References |
[1]. Clavulanic acid: a review. Biotechnol Adv. Jul-Aug 2008;26(4):335-51 [2]. Clavulanic acid, a novel inhibitor of beta-lactamases. Antimicrob Agents Chemother. 1978 Nov;14(5):650-5. [3]. In vitro activity and in vivo efficacy of clavulanic acid against Acinetobacter baumannii. Antimicrob Agents Chemother. 2009 Oct;53(10):4298-304. [4]. Tannic acid exhibits anti-inflammatory effects on formalin-induced paw edema model of inflammation in rats. Hum Exp Toxicol. 2019 Nov;38(11):1296-1301. |
| Additional Infomation |
Clavulanic acid is antibiotic isolated from Streptomyces clavuligerus. It acts as a suicide inhibitor of bacterial beta-lactamase enzymes. It has a role as an antibacterial drug, a bacterial metabolite, an anxiolytic drug and an EC 3.5.2.6 (beta-lactamase) inhibitor. It is a conjugate acid of a clavulanate. Clavulanic acid is a beta-lactamase inhibitor that is frequently combined with [Amoxicillin] or [Ticarcillin] to fight antibiotic resistance by preventing their degradation by beta-lactamase enzymes, broadening their spectrum of susceptible bacterial infections. Clavulanic acid is derived from the organism Streptomyces clavuligerus.When it is combined with amoxicillin, clavulanic acid is frequently known as Augmentin, Co-Amoxiclav, or Clavulin. Clavulanic acid is a beta Lactamase Inhibitor. The mechanism of action of clavulanic acid is as a beta Lactamase Inhibitor. Clavulanic acid has been reported in Streptomyces cattleya and Streptomyces clavuligerus with data available. Clavulanic Acid is a semisynthetic beta-lactamase inhibitor isolated from Streptomyces. Clavulanic acid contains a beta-lactam ring and binds strongly to beta-lactamase at or near its active site, thereby hindering enzymatic activity. This protects other beta-lactam antibiotics from beta-lactamase catalysis, thereby enhancing their antibacterial effects. This agent is used in conjunction with beta-lactamase susceptible antibiotics, such as penicillins and cephalosporins, to treat infections caused by beta-lactamase producing organisms. A beta-lactam antibiotic produced by the actinobacterium Streptomyces clavuligerus. It is a suicide inhibitor of bacterial beta-lactamase enzymes. Administered alone, it has only weak antibacterial activity against most organisms, but given in combination with other beta-lactam antibiotics it prevents antibiotic inactivation by microbial lactamase. Drug Indication Clavulanic acid combined with other antibiotics is indicated to prevent the development of drug-resistant strains of bacteria and promotes their therapeutic antibacterial effects. The following conditions, when they produced beta-lactamases, have been treated with a combination of amoxicillin and clavulanic acid or ticarcillin and clavulanic acid: Acute otitis media caused by H. influenzae and M. catarrhalis Sinusitis due to H. influenzae and M. catarrhalis Lower respiratory tract infections due to Haemophilus influenzae, S.aureus, Klebsiella species, and Moraxella catarrhalis Skin and skin structure infections caused by Staphylococcus aureus, Escherichia coli, and Klebsiella species Urinary Tract Infections due to E. coli, Klebsiella species of bacteria, and Enterobacter species of bacteria, S.marcescens, or S.aureus Gynecologic infections due to a variety of bacteria, including P.melaninogenicus, Enterobacter species, E.Coli species, Klebsiella species, S. aureus, S.epidermidis Septicemia due to a variety of bacteria, including Klebsiella species, E.Coli species, S.aureus, or Pseudomonas species Bone and joint infections due to S.aureus Intraabdominal infections due to E.Coli, K.pnemoniae, or B.fragilis group **A note on susceptibility** It should be noted that it is only to be administered in infections that are confirmed or highly likely to be caused by susceptible bacteria. Culture and susceptibility tests should be performed if possible and used in selecting whether this antibiotic is prescribed. When beta-lactamase enzyme production is not detected during microbiological testing, clavulanic acid should not be used. When these tests are not available patterns of local infection and susceptibility may be used to determine the appropriateness of using clavulanic acid. Ticarcillin with clavulanate has shown particular efficacy in mixed infections in addition to empiric therapy before determining the susceptibility of causative organisms. The ticarcillin-clavulanic acid combination may prove to be an effective single-agent antibiotic therapy to treat infections where a regimen of several drugs may normally be used. Mechanism of Action Clavulanic acid contains a beta-lactam ring in its structure that binds in an irreversible fashion to beta-lactamases, preventing them from inactivating certain beta-lactam antibiotics, with efficacy in treating susceptible gram-positive and gram-negative infections. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~13.89 mg/mL (~69.74 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (12.55 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (12.55 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: 10% DMSO+90% (20% SBE-β-CD in Saline): ≥ 2.5 mg/mL (12.55 mM) Solubility in Formulation 4: 12.5 mg/mL (62.76 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication (<60°C). (Please use freshly prepared in vivo formulations for optimal results.) |