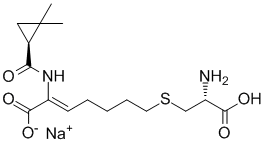
Cilastatin sodium (formerly known as MK-791; MK0791; Recarbrio), the sodium salt of Cilastatin, is a renal dehydropeptidase inhibitor, as well as a leukotriene D4 dipeptidase inhibitor with nephroprotective effects. Dehydropeptidase is an enzyme found in the kidney and is responsible for degrading the antibiotic imipenem. Cilastatin can therefore be combined intravenously with imipenem in order to protect it from dehydropeptidase and prolong its antibacterial effect. Cilastatin itself does not have antibiotic activity although it has been proved to be active against a zinc-dependent beta-lactamase that usually confer antibiotic resistance to certain bacteria.
Physicochemical Properties
| Molecular Formula | C16H25N2NAO5S |
| Molecular Weight | 380.4348 |
| Exact Mass | 380.138 |
| Elemental Analysis | C, 50.51; H, 6.62; N, 7.36; Na, 6.04; O, 21.03; S, 8.43 |
| CAS # | 81129-83-1 |
| Related CAS # | Cilastatin;82009-34-5 |
| PubChem CID | 6435415 |
| Appearance | Solid powder |
| Boiling Point | 655.5ºC at 760 mmHg |
| Flash Point | 350.2ºC |
| LogP | 1.189 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 11 |
| Heavy Atom Count | 24 |
| Complexity | 519 |
| Defined Atom Stereocenter Count | 2 |
| SMILES | C([C@H]1CC1(C)C)(=O)N/C(/C(=O)O)=C\CCCCSC[C@H](N)C(=O)O.[Na] |
| InChi Key | QXPBTTUOVWMPJN-QBNHLFMHSA-M |
| InChi Code | InChI=1S/C16H26N2O5S.Na/c1-16(2)8-10(16)13(19)18-12(15(22)23)6-4-3-5-7-24-9-11(17)14(20)21/h6,10-11H,3-5,7-9,17H2,1-2H3,(H,18,19)(H,20,21)(H,22,23)/q+1/p-1/b12-6-/t10-,11+/m1./s1 |
| Chemical Name | sodium S-((Z)-6-carboxy-6-((S)-2,2-dimethylcyclopropane-1-carboxamido)hex-5-en-1-yl)-L-cysteinate |
| Synonyms | Cilastatin Monosodium Salt ; MK 0791; MK0791; M K-0791; MK791; MK-791; MK-791; Cilastatin sodium; Recarbrio; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: (1). This product requires protection from light (avoid light exposure) during transportation and storage.(2). Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam |
| ln Vitro | Without lessening the antimicrobial effect of Vancomycin, ciplastatin (200 μg/mL; 24 hours; RPTECs) treatment prevents Vancomycin-induced proximal tubule apoptosis and boosts cell viability[2]. |
| ln Vivo | Ipenem and cilastatin together protected mice against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa infection in a mouse model of systemic infection (female mice, CD-1 strain, 20 g) [3]. |
| Cell Assay |
Cell Line: Renal proximal tubular epithelial cells (RPTECs) Concentration: 200 μg/mL Incubation Time: 24 hours Result: Significantly ameliorated Vancomycin-induced nuclear apoptosis. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Cilastatin is reported by official FDA labeling to be 70% excreted in the urine, however published literature has reported values as high as 98%. Cilastatin has a volume of distribution of 14.6-20.1L. Cilastatin has a total clearance of 0.2 L/h/kg and a renal clearance of 0.10-0.16 L/h/kg. Biological Half-Life The half-life of cilastatin is approximately 1h. |
| Toxicity/Toxicokinetics |
Protein Binding Cilastatin is plasma protein binding is reported to be 35-40%. |
| References |
[1]. Keynan S, et al. The renal membrane dipeptidase (dehydropeptidase I) inhibitor, cilastatin, inhibits the bacterialmetallo-beta-lactamase enzyme CphA. Antimicrob Agents Chemother. 1995 Jul;39(7):1629-31. [2]. S Keynan, et al. The Renal Membrane Dipeptidase (Dehydropeptidase I) Inhibitor, Cilastatin, Inhibits the Bacterial Metallo-Beta-Lactamase Enzyme CphA. Antimicrob Agents Chemother. 1995 Jul;39(7):1629-31. [3]. Blanca Humanes, et al. Protective Effects of Cilastatin Against Vancomycin-Induced Nephrotoxicity. Biomed Res Int. 2015;2015:704382. [4]. P J Petersen, et al. In Vitro and in Vivo Activities of LJC10,627, a New Carbapenem With Stability to Dehydropeptidase I. Antimicrob Agents Chemother. 1991 Jan;35(1):203-7. |
| Additional Infomation |
Cilastatin is the thioether resulting from the formal oxidative coupling of the thiol group of L-cysteine with the 7-position of (2Z)-2-({[(1S)-2,2-dimethylcyclopropyl]carbonyl}amino)hept-2-enoic acid. It is an inhibitor of dehydropeptidase I (membrane dipeptidase, 3.4.13.19), an enzyme found in the brush border of renal tubes and responsible for degrading the antibiotic imipenem. Cilastatin is therefore administered (as the sodium salt) with imipenem to prolong the antibacterial effect of the latter by preventing its renal metabolism to inactive and potentially nephrotoxic products. Cilastatin also acts as a leukotriene D4 dipeptidase inhibitor, preventing the metabolism of leukotriene D4 to leukotriene E4. It has a role as a protease inhibitor, an EC 3.4.13.19 (membrane dipeptidase) inhibitor, a xenobiotic and an environmental contaminant. It is a non-proteinogenic L-alpha-amino acid, a L-cysteine derivative, an organic sulfide and a carboxamide. It is a conjugate acid of a cilastatin(1-). Cilastatin is an inhibitor of renal dehydropeptidase, an enzyme responsible for both the metabolism of thienamycin beta-lactam antibiotics as well as conversion of leukotriene D4 to leukotriene E4. Since the antibiotic, [imipenem], is one such antibiotic that is hydrolyzed by dehydropeptidase, cilastatin is used in combination with imipenem to prevent its metabolism. The first combination product containing both drugs was approved by the FDA in November of 1985 under the trade name Primaxin, marketed by Merck & Co. A newer triple-drug product was approved in July 2019 under the trade name Recarbrio which also contains [relebactam]. Cilastatin is a Renal Dehydropeptidase Inhibitor. The mechanism of action of cilastatin is as a Dipeptidase Inhibitor. Cilastatin has been reported in Bos taurus and Apis cerana with data available. A renal dehydropeptidase-I and leukotriene D4 dipeptidase inhibitor. Since the antibiotic, IMIPENEM, is hydrolyzed by dehydropeptidase-I, which resides in the brush border of the renal tubule, cilastatin is administered with imipenem to increase its effectiveness. The drug also inhibits the metabolism of leukotriene D4 to leukotriene E4. See also: ... View More ... Drug Indication Cilastatin is indicated, in combination with [imipenem] with or without [relebactam], for the treatment of bacterial infections including respiratory, skin, bone, gynecologic, urinary tract, and intra-abdominal as well as septicemia and endocarditis. FDA Label Mechanism of Action Cilastatin is a renal dehydropeptidase-I inhibitor. Since the antibiotic, imipenem, is hydrolyzed by dehydropeptidase-I, which resides in the brush border of the renal tubule, cilastatin is administered with imipenem to block the metabolism of imipenem. Pharmacodynamics Cilastatin is a chemical compound which inhibits the human enzyme dehydropeptidase. Renal Dehydropeptidase degrades the antibiotic [imipenem]. Cilastatin is therefore combined intravenously with imipenem in order to protect it from dehydropeptidase and prolong its antibacterial effect. However, cilastatin in and of itself does not have any antibacterial activity. The increased renal excretion of unchanged imipenem appears to prevent proximal tubular necrosis associated with high doses of imipenem. |
Solubility Data
| Solubility (In Vitro) |
DMSO : ~72 mg/mL ( ~200.86 mM ) Water : 7~100 mg/mL(~262.86 mM ) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6286 mL | 13.1430 mL | 26.2860 mL | |
| 5 mM | 0.5257 mL | 2.6286 mL | 5.2572 mL | |
| 10 mM | 0.2629 mL | 1.3143 mL | 2.6286 mL |