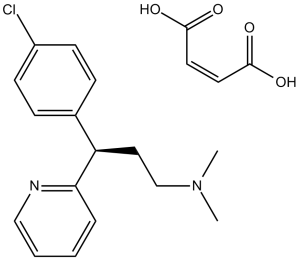
Chlorpheniramine Maleate (Piriton; Chlortrimeton; Deconamine; Neorestamin; NCIC-55265; NCIC55265; Chlo-Amine; Chlor-100; Chlor-Trimeton), the Maleate salt of Chlorpheniramine, is a potent and 1st-generation alkylamine-based histamine H1 receptor antagonist with anti-allergic effects. It suppresses the histamine H1 receptor with an IC50 of 12 nM. Chlorpheniramine has been extensively employed in the management and avoidance of allergic reaction symptoms, including urticaria and rhinitis.
Physicochemical Properties
| Molecular Formula | C20H23CLN2O4 | |
| Molecular Weight | 390.86 | |
| Exact Mass | 390.134 | |
| Elemental Analysis | C, 61.46; H, 5.93; Cl, 9.07; N, 7.17; O, 16.37 | |
| CAS # | 113-92-8 | |
| Related CAS # | Chlorpheniramine; 132-22-9; Chlorpheniramine-d4 maleate; 2747915-71-3 | |
| PubChem CID | 2725 | |
| Appearance | White crystalline solid | |
| Density | 1.1±0.1 g/cm3 | |
| Boiling Point | 379.0±42.0 °C at 760 mmHg | |
| Melting Point | 130-135 °C(lit.) | |
| Flash Point | 183.0±27.9 °C | |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C | |
| Index of Refraction | 1.565 | |
| LogP | 3.39 | |
| Hydrogen Bond Donor Count | 0 | |
| Hydrogen Bond Acceptor Count | 2 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 19 | |
| Complexity | 249 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | ClC1C([H])=C([H])C(=C([H])C=1[H])C([H])(C1=C([H])C([H])=C([H])C([H])=N1)C([H])([H])C([H])([H])[N+]([H])(C([H])([H])[H])C([H])([H])[H].O([H])C(/C(/[H])=C(/[H])\C(=O)[O-])=O |
|
| InChi Key | DBAKFASWICGISY-BTJKTKAUSA-N | |
| InChi Code | InChI=1S/C16H19ClN2.C4H4O4/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13;5-3(6)1-2-4(7)8/h3-9,11,15H,10,12H2,1-2H3;1-2H,(H,5,6)(H,7,8)/b;2-1- | |
| Chemical Name | (Z)-but-2-enedioic acid;3-(4-chlorophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | H1 Receptor ( IC50 = 12 nM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | Segments of isolated ileum, measuring 1 centimeter, are suspended in an organ bath that contains Tyrode solution at 32 degrees Celsius (ventilation). Using an isotonic transducer, the contractile reactions to histamine (0.54 μM) are measured. Five minutes prior to the addition of histamine, an organ bath is filled with a predetermined concentration of chlorpheniramine. The probit method is used to determine the IC50 value of chlorpheniramine. | ||
| Cell Assay | For 48 hours, cells are exposed to different chlorpheniramine concentrations. To assess cell growth, cells are cleaned, separated, and counted using a Coulter counter. | ||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Well absorbed in the gastrointestinal tract. STUDIES IN MAN & EXPTL ANIMALS INDICATE THAT (3)H-CHLORPHENIRAMINE MALEATE IS RAPIDLY & QUANT ABSORBED FROM GUT. ALTHOUGH PLASMA LEVELS OF TOTAL RADIOACTIVITY ARE PROLONGED, PLASMA T/2 OF CHLORPHENIRAMINE IS ONLY 12-15 HR IN MAN & 3 HR IN DOG. T/2 IN MAN IS ABOUT 3 TIMES LONGER THAN THERAPEUTIC EFFECT... The H1 antagonists are well absorbed from the GI tract. Following oral administration, peak plasma concn are achieved in 2 to 3 hr and effects usually last 4 to 6 hr; however, some of the drugs are much longer acting ... . /Histamine Antagonists: H1 Antagonists/ H1 blockers are among the many drugs that induce hepatic microsomal enzymes, and they may facilitate their own metabolism. /Histamine Antagonists: H1 Antagonists/ Metabolism / Metabolites Primarily hepatic via Cytochrome P450 (CYP450) enzymes. MAIN SITE OF METABOLIC TRANSFORMATION IS LIVER. /ANTIHISTAMINES/ Primarily hepatic via Cytochrome P450 (CYP450) enzymes. Half Life: 21-27 hours Biological Half-Life 21-27 hours IN MAN...PLASMA T/2 OF CHLORPHENIRAMINE IS...12-15 HR...ALTHOUGH PLASMA LEVELS OF TOTAL RADIOACTIVITY ARE PROLONGED... Elimination: 14 to 25 hours |
||
| Toxicity/Toxicokinetics |
Toxicity Summary Chlorpheniramine binds to the histamine H1 receptor. This blocks the action of endogenous histamine, which subsequently leads to temporary relief of the negative symptoms brought on by histamine. Toxicity Data Oral LD50 (rat): 306 mg/kg Oral LD50 (mice): 130 mg/kg Oral LD50 (guinea pig): 198 mg/kg LD50: 306 mg/kg (Human) (A308) Interactions Concurrent use /of ototoxic medications/ with antihistamines may mask the symptoms of ototoxicity such as tinnitus, dizziness, or vertigo. /Antihistamines/ Concurrent use of monoamine oxidase (MAO) inhibitors with antihistamines may prolong and intensify the anticholinergic and CNS depressant effects of antihistamines; concurrent use is not recommended. /Antihistamines/ Concurrent use /with alcohol or other CNS depression-producing medications/ may potentiate the CNS depressant effects of either these medications or antihistamines; also, concurrent use of maprotiline or tricyclic antidepressants may potentiate the anticholinergic effects of either antihistamines or these medications. /Antihistamines/ Anticholinergic effects may be potentiated when /anticholinergics or other medications with anticholinergic activity/ are used concurrently with antihistamines; patients should be advised to report occurrence of gastrointestinal problems promptly since paralytic ileus may occur with concurrent therapy. /Antihistamines/ Concurrent use /of other photosensitizing medications/ with antihistamines may cause additive photosensitizing effects. /Antihistamines/ |
||
| References |
[1]. J Med Chem . 1986 Jul;29(7):1178-83. [2]. J Med Chem . 1991 Apr;34(4):1314-28. [3]. Breast Cancer Res Treat . 1995 Aug;35(2):187-94. [4]. Antimicrob Agents Chemother . 2007 Nov;51(11):4133-40. [5]. J Pharmacol Exp Ther . 2009 Aug;330(2):403-12. [6]. Eur J Pharmacol . 2003 Jun 27;471(3):223-8. |
||
| Additional Infomation |
Therapeutic Uses Anti-Allergic Agents; Antipruritics; Histamine H1 Antagonists Antihistamines are indicated in the prophylactic and symptomatic treatment of perennial and seasonal allergic rhinitis, vasomotor rhinitis, and allergic conjunctivitis due to inhalant allergens and foods. /Antihistamines; Included in US product labeling/ Antihistamines are indicated for the symptomatic treatment of pruritus associated with allergic reactions and of mild, uncomplicated allergic skin manifestations of urticaria and angioedema, in dermatographism, and in urticaria associated with transfusions. /Antihistamines; Included in US product labeling/ Antihistamines are also used in the treatment of pruritus associated with pityriasis rosea. /Antihistamines; NOT included in US product labeling/ For more Therapeutic Uses (Complete) data for CHLORPHENIRAMINE (10 total), please visit the HSDB record page. Drug Warnings Use is not recommended in newborn or premature infants because this age group has an increased susceptibility to anticholinergic side effects, such as central nervous system excitation, and an increased tendency toward convulsions. A paradoxical reaction characterized by hyperexcitability may occur in children taking antihistamines. /Antihistamines/ Dizziness, sedation, confusion, and hypotension may be more likely to occur in geriatric patients taking antihistamines. Geriatric patients are especially susceptible to the anticholinergic side effects, such as dryness of mouth and urinary retention (especially in males), of the antihistamines. If these side effects occur and continue or are severe, medication should probably be discontinued. /Antihistamines/ Prolonged use of antihistamines ... may decrease or inhibit salivary flow, thus contributing to the development of caries, periodontal disease, oral candidiasis, and discomfort. /Antihistamines/ ANTIHISTAMINE DRUGS MAY BE OF SOME USE IN MINIMIZING SERUM REACTIONS BUT ARE OF NO THERAPEUTIC VALUE...& MAY EVEN POTENTIATE TOXIC ACTION OF VENOM... /ANTIHISTAMINES/ For more Drug Warnings (Complete) data for CHLORPHENIRAMINE (14 total), please visit the HSDB record page. Pharmacodynamics In allergic reactions an allergen interacts with and cross-links surface IgE antibodies on mast cells and basophils. Once the mast cell-antibody-antigen complex is formed, a complex series of events occurs that eventually leads to cell-degranulation and the release of histamine (and other chemical mediators) from the mast cell or basophil. Once released, histamine can react with local or widespread tissues through histamine receptors. Histamine, acting on H1-receptors, produces pruritis, vasodilatation, hypotension, flushing, headache, tachycardia, and bronchoconstriction. Histamine also increases vascular permeability and potentiates pain. Chlorpheniramine, is a histamine H1 antagonist (or more correctly, an inverse histamine agonist) of the alkylamine class. It competes with histamine for the normal H1-receptor sites on effector cells of the gastrointestinal tract, blood vessels and respiratory tract. It provides effective, temporary relief of sneezing, watery and itchy eyes, and runny nose due to hay fever and other upper respiratory allergies. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.40 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.40 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (6.40 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: Saline: 20 mg/mL Solubility in Formulation 5: 120 mg/mL (307.02 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5585 mL | 12.7923 mL | 25.5846 mL | |
| 5 mM | 0.5117 mL | 2.5585 mL | 5.1169 mL | |
| 10 mM | 0.2558 mL | 1.2792 mL | 2.5585 mL |