Physicochemical Properties
| Molecular Formula | C11H12CL2N2O5 |
| Molecular Weight | 323.13 |
| Exact Mass | 322.012 |
| Elemental Analysis | C, 40.89; H, 3.74; Cl, 21.94; N, 8.67; O, 24.76 |
| CAS # | 56-75-7 |
| Related CAS # | Chloramphenicol-d5;202480-68-0;Chloramphenicol palmitate;530-43-8;Levomecol;118573-58-3;DL-threo-Chloramphenicol-d5;1420043-66-8;Threo-Chloramphenicol-d6;Chloramphenicol-d4 |
| PubChem CID | 5959 |
| Appearance | White to off-white crystalline powder. |
| Density | 1.6±0.1 g/cm3 |
| Boiling Point | 563.2±60.0 °C at 760 mmHg |
| Melting Point | 148-150 °C(lit.) |
| Flash Point | 294.4±32.9 °C |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.623 |
| LogP | 1.62 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 20 |
| Complexity | 342 |
| Defined Atom Stereocenter Count | 2 |
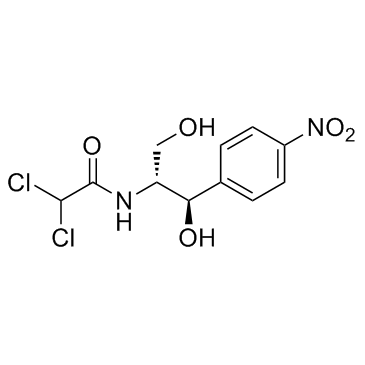
| SMILES | ClC([H])(C(N([H])[C@]([H])(C([H])([H])O[H])[C@@]([H])(C1C([H])=C([H])C(=C([H])C=1[H])[N+](=O)[O-])O[H])=O)Cl |
| InChi Key | WIIZWVCIJKGZOK-RKDXNWHRSA-N |
| InChi Code | InChI=1S/C11H12Cl2N2O5/c12-10(13)11(18)14-8(5-16)9(17)6-1-3-7(4-2-6)15(19)20/h1-4,8-10,16-17H,5H2,(H,14,18)/t8-,9-/m1/s1 |
| Chemical Name | 2,2-dichloro-N-[(1R,2R)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide |
| Synonyms | Chloramphenicol; Chlornitromycin; Chloromycetin; Levomycetin; Chlorocid; Globenicol; Detreomycin; Kloramfenikol; Levomycetin; Ophthochlor; Syntomycin; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product is not stable in solution, please use freshly prepared working solution for optimal results. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | JNK;MMP13 |
| ln Vitro |
The HIF-1α pathway in NSCLC cells is inhibited by chloramphenicol (1-100 μg/mL, 18-24 h) in a concentration-dependent manner.NSCLC cells are exposed to 100 μg/mL of chloramphenicol for 0–24 hours, which causes autophagy induction and significantly raises the levels of autophagic biomarkers (beclin-1, Atg12–Atg5 conjugates, and LC3–II)[1]. In activated T cells, chloramphenicol inhibits apoptosis and causes aberrant differentiation[2]. Chloramphenicol can cause reduced ATP biosynthesis and mitochondrial stress by blocking the synthesis of proteins in both bacteria and mitochondria[3]. Chloramphenicol (1-100 μg/mL) has the ability to upregulate MMP-13 protein and stimulate the expression of matrix metalloproteinase (MMP)-13[3]. Chloramphenicol (1-100 μg/mL) has the ability to stimulate PI-3K/Akt signaling, c-Jun protein phosphorylation, and c-Jun N-terminal kinases (JNK)[3]. By inhibiting peptidyl transferase activity, chloramphenicol mainly affects the 50S subunit of bacterial 70S rihosomes, which prevents the formation of peptide bonds[5]. |
| ln Vivo | Day 1 post-dosing sees a decrease in marrow erythroid cells and erythrocyte precursors, and by day 14, after 14 days of treatment, erythrocytes and erythrocyte precursors have returned to normal[4]. |
| Animal Protocol |
Animal Model: Female B6C3F1 mice (12-14 weeks old) Dosage: 0, 2500 and 3500 mg/kg Administration: Gavage, daily, for 5 days Result: On the first day after dosage, erythropoiesis was clearly stopped. At the 2500 mg/kg dose level on day 7 and between 7 and 14 days at the 3500 mg/kg dose level, respectively, a recovery was observed after the dosage. At every dosage level, the erythroid series showed the greatest myelotoxicity. Day 1 post-dosage: decreased femoral marrow BFU-E and CFU-E. Within 14 days of the dosage, every blood and marrow parameter in the current study was back to normal. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Rapidly and completely absorbed from gastrointestinal tract following oral administration (bioavailability 80%). Well absorbed following intramuscular administration (bioavailability 70%). Intraocular and some systemic absorption also occurs after topical application to the eye. Hepatic metabolism to the inactive glucuronide is the major route of elimination. This metabolite and chloramphenicol itself are excreted in the urine following filtration and secretion. Chloramphenicol administered orally is absorbed rapidly from the intestinal tract. In controlled studies in adult volunteers using the recommended dosage of 50 mg/kg/day, a dosage of 1 g every 6 hours for 8 doses was given. Using the microbiological assay method, the average peak serum level was 11.2 ug/mL one hour after the first dose. A cumulative effect gave a peak rise to 18.4 ug/mL after the fifth dose of 1 g. Mean serum levels ranged from 8 to 14 ug/mL over the 48-hour period. Total urinary excretion of chloramphenicol in these studies ranged from a low of 68% to a high of 99% over a three-day period. From 8% to 12% of the antibiotic excreted is in the form of free chloramphenicol; the remainder consists of microbiologically inactive metabolites, principally the conjugate with glucuronic acid. Since the glucuronide is excreted rapidly, most chloramphenicol detected in the blood is in the microbiologically active free form. Despite the small proportion of unchanged drug excreted in the urine, the concentration of free chloramphenicol is relatively high, amounting to several hundred mcg/mL in patients receiving divided doses of 50 mg/kg/day. Small amounts of active drug are found in bile and feces. Chloramphenicol diffuses rapidly, but its distribution is not uniform. Highest concentrations are found in liver and kidney, and lowest concentrations are found in brain and cerebrospinal fluid. Chloramphenicol enters cerebrospinal fluid even in the absence of meningeal inflammation, appearing in concentrations about half of those found in the blood. Measurable levels are also detected in pleural and in ascitic fluids, saliva, milk, and in the aqueous and vitreous humors. Transport across the placental barrier occurs with somewhat lower concentration in cord blood of neonates than in maternal blood. Chloramphenicol achieves maximum serum levels very rapidly following oral, intravenous and intraperitoneal administration. Intramuscular injection with chloramphenicol, except certain soluble forms, results in a somewhat delayed absorption and lower serum levels than when given by the oral, intravenous, or intraperitoneal route. Chloramphenicol diffuses readily into all body tissues, but at different concentrations. Highest concentrations are found in the liver and kidney of dogs indicating that these organs are the main route of inactivation and excretion of the metabolites. The lungs, spleen, heart and skeletal muscles contain concentrations similar to that of the blood. Chloramphenicol reaches significant concentration in the aqueous and vitreous humors of the eye from the blood. A significant difference from other antibiotics is its marked ability to diffuse into the cerebrospinal fluid. Within three to four hours after administration, the concentration in the cerebrospinal fluid has reached, on the average, 50% of the concentration in the serum. If the meninges are inflamed, the percentage may be even higher. Chloramphenicol diffuses readily into milk, pleural and ascitic fluids and crosses the placenta attaining concentrations of about 75% of that of the maternal blood. Approximately 55% of a single daily dose can be recovered from the urine of a treated dog. A small fraction of this is in the form of unchanged chloramphenicol. For more Absorption, Distribution and Excretion (Complete) data for Chloramphenicol (21 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic, with 90% conjugated to inactive glucuronide. Chloramphenicol is rather rapidly metabolized, mainly in the liver, by conjugation with glucuronic acid. Yields d-threo-2-amino-1-(p-nitrophenyl)-1,3-propanediol and chloramphenicol-beta-d-glucuronide in man. In rat. /from table/ ... /undergoes/ direct conjugation. Formation of glucuronide was shown to occur at primary rather than at secondary alcoholic group ... its major reaction of inactivation and detoxication of drug in man, and any factor which decreases its importance ... results in greatly increased toxicity. ... In newborns ... bilirubin ... acts as competitive endogenous acceptor. Chloramphenicol 3-glucuronide was the major metabolite of chloramphenicol produced by isolated rat hepatocytes although a minor metabolite was also formed. For more Metabolism/Metabolites (Complete) data for Chloramphenicol (10 total), please visit the HSDB record page. Biological Half-Life Half-life in adults with normal hepatic and renal function is 1.5 - 3.5 hours. In patients with impaired renal function half-life is 3 - 4 hours. In patients with severely impaired hepatic function half-life is 4.6 - 11.6 hours. Half-life in children 1 month to 16 years old is 3 - 6.5 hours, while half-life in infants 1 to 2 days old is 24 hours or longer and is highly variable, especially in low birth-weight infants. Chloramphenicol has a half-time /in humans/ ranging from 1.6 to 4.6 hr; using different techniques and in different adult patients, apparent volumes of distribution ranging from 0.2 to 3.1 l/kg have been measured ... . The half-time is considerably longer in neonates ... in 1- to 8-day-old infants the half-life ranged from 10 to over 48 hr, and in 11-day to 8-wk-old infants the range was 5-16 hr ... . The plasma half-life of chloramphenicol in adults with normal renal and hepatic function is 1.5-4.1 hours. ... The plasma half-life is 24 hours or longer in infants 1-2 days of age and approximately 10 hours in infants 10-16 days of age. The plasma half-life of chloramphenicol is prolonged in patients with markedly reduced hepatic function. In patients with impaired renal function, the plasma half-life of chloramphenicol is not significantly prolonged, although half-lives of the inactive conjugated derivatives may be prolonged. |
| Toxicity/Toxicokinetics |
Toxicity Summary IDENTIFICATION AND USE: Chloramphenicol is an antibiotic for human and veterinary use, originally isolated from the soil bacterium Streptomyces venzeuelae. HUMAN STUDIES: Chloramphenicol is known to produce major adverse effects in humans. One of these is generally irreversible aplastic anemia and reversible bone marrow suppression. A link between chloramphenicol and liver disease has been noted. Acute myeloid leukemia is also associated with chloramphenicol exposure. Neonates, especially if premature, may develop a serious illness termed "gray baby syndrome" if exposed to excessive doses of chloramphenicol. This syndrome usually begins 2-9 days (average of 4 days) after treatment is started. Within the first 24 hours, vomiting, refusal to suck, irregular and rapid respiration, abdominal distention, periods of cyanosis, and passage of loose green stools occur. The children are severely ill by the end of the first day, and in the next 24 hours turn an ashen-gray color and become flaccid and hypothermic. A similar "gray syndrome" has been reported in adults who were accidentally overdosed with the drug. Allergic contact dermatitis has been noted from a patient using a topical cream containing chloramphenicol. Optic neuritis and scotoma with failing vision has also been reported. Chloramphenicol has been implicated in cleft lip. Chromosomal anomalies were noted in human lymphocytes treated with chloramphenicol in vitro. ANIMAL STUDIES: After three groups of ten 3-month old mice were given daily intraperitoneal injections of chloramphenicol at 20, 40 or 100 mg/kg bw for 3 months, splenomegaly, hepatomegaly, lymph adenopathy and hypertrophy of the thymus occurred in a dose-dependent fashion. An increased incidence in lymphomas in two strains of mice were noted after exposure to chloramphenicol in drinking water. Chloramphenicol has been shown to cause DNA strand breaks in bacterial cells and to inhibit DNA synthesis in lymphocytes and in the phage of Escherichia coli. Chromosomal anomalies were noted in mouse bone marrow cells given chloramphenicol ip or im in F1 generation mouse liver. When groups of 3-9 female Sprague-Dawley rats were given chloramphenicol in drinking water with or without exposure to short duration high intensity noise, ototoxicity was noted. Oral doses of this antibiotic suppressed paradoxical sleep in cats. Chloramphenicol administered to pregnant monkeys had no effect on fetal development. High oral doses of chloramphenicol of 500-2000 mg/kg to rats and mice and of 500 and 1000 mg/kg to rabbits produced high incidences of embryonic and fetal deaths and fetal growth retardation in all three species. Teratogenic effects, predominantly umbilical hernia, were observed only in rats. The pregnant animals showed no toxic sign, except that those given the highest dose gained significantly less weight than controls. Progeny of chloramphenicol-treated mice had reduced learning ability, higher brain seizure threshold and poorer performance in the open-field test. ECOTOXICITY STUDIES: Chloramphenicol was added to cultures of one freshwater green alga, Chlorella pyrenoidosa, and two marine algae, Isochrysis galbana and Tetraselmis chui. It was more toxic to the freshwater algae. Chloramphenicol exposure could significantly inhibit the growth of Scenedesmus obliquus, while Chlorella pyrenoidosa exhibited less sensitivity. Abnormal behavioral changes were observed in fish Clarias gariepinus exposed to chloramphenicol. Hematological parameters were also affected. In Egyptian toads (Bufo regularis) given a dose of chloramphenicol of 5 mg/40 g body weight for 12 weeks, it induced numerous, severe ultrastructural changes in almost all types of leukocytes. Hepatotoxicity A proportion of patients with blood dyscrasias due to chloramphenicol also developed clinically apparent liver injury with jaundice, usually occurring before the appearance of aplastic anemia or severe thrombocytopenia. Jaundice arises in 10% to 25% of cases of aplastic anemia, usually within 1 to 2 months of starting chloramphenicol and often shortly after it is stopped. Aplastic anemia and the accompanying liver injury occur most frequently in patients who receive multiple courses of chloramphenicol or prolonged therapy. The serum enzyme pattern is usually hepatocellular and the clinical presentation is an acute hepatitis-like syndrome with onset of fatigue, nausea, anorexia and abdominal discomfort followed by dark urine and jaundice. Rare instances have a cholestatic pattern of presentation with jaundice and itching and prominent elevations in alkaline phosphatase. Some cases occur in the absence of bone marrow involvement. Immunoallergic and autoimmune features are rarely present. The course is self-limited in most instances, but examples of acute liver failure have been reported, particularly in patients without aplastic anemia. In most cases, however, the liver injury associated with chloramphenicol use is eclipsed by the severe bone marrow aplasia. Likelihood score: B (highly likely cause of clinically apparent liver injury, now rarely seen). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Adverse reactions such as vomiting, excessive intestinal gas and falling asleep at the breast have been reported in breastfed infants whose mothers were taking oral chloramphenicol. Milk concentrations are not sufficient to induce "gray baby" syndrome, but since chloramphenicol-induced aplastic anemia is not dose-related, this might occur, but has not been reported. An alternate drug is preferred to chloramphenicol during breastfeeding, especially while nursing a newborn or preterm infant. If the mother must receive chloramphenicol during nursing, monitor the infant for gastrointestinal disturbances and adequacy of nursing. Monitoring of the infant's complete blood count and differential is advisable. In some cases, discontinuation of breastfeeding might be preferred. ◉ Effects in Breastfed Infants One study reported 50 breastfed infants whose mothers were give oral chloramphenicol beginning 2 to 12 days postpartum in dosages of 1 (n = 20), 2 (n = 20)or 3 grams (n = 10) daily. All of the infants refused to suck, and 50 to 60% fell asleep during nursing. Vomiting occurred after feeding in 10%, 25%, and 90% of infants with daily maternal dosages of 1, 2 and 3 grams, respectively. All infants had excessive intestinal gas and abdominal distention, with severe problems in 0.5%, 20% and 100% of infants with daily maternal dosages of 1, 2 and 3 grams, respectively. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Plasma protein binding is 50-60% in adults and 32% is premature neonates. Interactions Chloramphenicol-treated patient may respond poorly to cyanocobalamin therapy since chloramphenicol interferes with erythrocyte maturation. Concurrent therapy with other drugs that may cause bone marrow depression should be avoided. Chloramphenicol antagonizes the action of such antibiotics as penicillin and streptomycin, which act only on growing cells, but is synergistic to tetracycline, which also acts by inhibiting protein synthesis. It is possible the chloramphenicol would produce similar synergism with other antibiotics which act by inhibiting protein synthesis. Clinical observations in man and corresponding investigations in laboratory animals experimentally infected with various pathogenic bacteria have shown that a combination of chloramphenicol with gamma-globulin or specific antisera has a greater therapeutic effect than would be expected from a mere addition of the individual effects. For more Interactions (Complete) data for Chloramphenicol (26 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat ip 1811 mg/kg LD50 Rat sc 5 g/kg LD50 Rat iv 171 mg/kg LD50 Mouse oral 1500 mg/kg For more Non-Human Toxicity Values (Complete) data for Chloramphenicol (14 total), please visit the HSDB record page. |
| References |
[1]. Chloramphenicol Induces Autophagy and Inhibits the Hypoxia Inducible Factor-1 Alpha Pathway in Non-Small Cell Lung Cancer Cells. Int J Mol Sci. 2019 Jan 3;20(1):157. [2]. Chloramphenicol induces abnormal differentiation and inhibits apoptosis in activated T cells. Cancer Res. 2008 Jun 15;68(12):4875-81. [3]. Chloramphenicol causes mitochondrial stress, decreases ATP biosynthesis, induces matrix metalloproteinase-13 expression, and solid-tumor cell invasion. Toxicol Sci. 2010 Jul;116(1):140-50. [4]. Characterization of the myelotoxicity of chloramphenicol succinate in the B6C3F1 mouse. Int J Exp Pathol. 2006 Apr;87(2):101-12. [5]. Jardetzky, O., Studies on the mechanism of action of chloramphenicol. I. The conformation of chlioramphenicol in solution. J Biol Chem, 1963. 238: p. 2498-508. [6]. Wolfe, A.D. and F.E. Hahn, Mode of Action of Chloramphenicol. Ix. Effects of Chloramphenicol Upon a Ribosomal Amino Acid Polymerization System and Its Binding to Bacterial Ribosome. Biochim Biophys Acta, 1965. 95: p. 146-55 |
| Additional Infomation |
Therapeutic Uses Anti-Bacterial Agents; Protein Synthesis Inhibitors /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Chloramphenicol is included in the database. Chloramphenicol is an antibiotic produced by Streptomyces venezuelae ... recommended for serious infections in which the location of the infection, susceptibility of the pathogen or poor response to other therapy indicate restricted antimicrobial option. It has been used since the 1950s for a wide range of microbial infections, including typhoid fever and other forms of salmonellosis, and central nervous system, anaerobic and ocular infections ... . (VET): Chloramphenicol Tablets are recommended for oral treatment of the following conditions in dogs: Bacterial pulmonary infections caused by susceptible microorganisms such as: Staphylococcus aureus, Streptococcus pyogenes and Brucella bronchiseptica; infections of the urinary tract caused by susceptible microorganisms such as: Escherichia coli, Proteus vulgaris, Corynebacterium renale, Streptococcus spp., and hemolytic Staphylococcus; enteritis caused by susceptible microorganisms such as: E. coli, Proteus spp., Salmonella spp., and Pseudomonas spp.; infections associated with canine distemper caused by susceptible microorganims such as: B. bronchiseptica, E. coli, P. aeruginosa, Proteus spp., Shigella spp. and Neisseria catarrhalis. Additional adjunctive therapy should be used when indicated. Most susceptible infectious disease organisms will respond to chloramphenicol therapy in three to five days when the recommended dosage regimen is followed. If no response to chloramphenicol therapy is obtained in three to five days, discontinue its use and review the diagnosis. Also, a change of therapy should be considered. Laboratory tests should be conducted including in vitro culturing and susceptibility tests on samples collected prior to treatment. For more Therapeutic Uses (Complete) data for Chloramphenicol (38 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING Serious and fatal blood dyscrasias (aplastic anemia, hypoplastic anemia, thrombocytopenia and granulocytopenia) are known to occur after the administration of chloramphenicol. In addition, there have been reports of aplastic anemia attributed to chloramphenicol which later terminated in leukemia. Blood dyscrasias have occurred after both short-term and prolonged therapy with this drug. Chloramphenicol must not be used when less potentially dangerous agents will be effective, as described in the INDICATIONS AND USAGE section. It must not be used in the treatment of trivial infections or where it is not indicated, as in colds, influenza, infections of the throat; or as a prophylactic agent to prevent bacterial infections. Precautions: It is essential that adequate blood studies be made during treatment with the drug. While blood studies may detect early peripheral blood changes, such as leukopenia, reticulocytopenia, or granulocytopenia, before they become irreversible, such studies cannot be relied on to detect bone marrow depression prior to development of aplastic anemia. To facilitate appropriate studies and observation during therapy, it is desirable that patients be hospitalized. /BOXED WARNING/ WARNING Bone marrow hypoplasia including aplastic anemia and death has been reported following topical application of chloramphenicol. Chloramphenicol should not be used when less potentially dangerous agents would be expected to provide effective treatment. /Chloramphenicol/ is not recommended for the routine treatment of the typhoid carrier state. Chloramphenicol is contraindicated in individuals with a history of previous hypersensitivity and/or toxic reaction to it. It must not be used in the treatment of trivial infections or where it is not indicated, as in colds, influenza, infections of the throat; or as a prophylactic agent to prevent bacterial infections. For more Drug Warnings (Complete) data for Chloramphenicol (44 total), please visit the HSDB record page. Pharmacodynamics Chloramphenicol is a broad-spectrum antibiotic that was derived from the bacterium Streptomyces venezuelae and is now produced synthetically. Chloramphenicol is effective against a wide variety of microorganisms, but due to serious side-effects (e.g., damage to the bone marrow, including aplastic anemia) in humans, it is usually reserved for the treatment of serious and life-threatening infections (e.g., typhoid fever). Chloramphenicol is bacteriostatic but may be bactericidal in high concentrations or when used against highly susceptible organisms. Chloramphenicol stops bacterial growth by binding to the bacterial ribosome (blocking peptidyl transferase) and inhibiting protein synthesis. |
Solubility Data
| Solubility (In Vitro) |
DMSO :65~150 mg/mL (201.15~464.21 mM ) Ethanol : ~100 mg/mL (~309.47 mM ) H2O : ~3.06 mg/mL (~9.47 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.74 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.74 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (7.74 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: ≥ 2.5 mg/mL (7.74 mM) (saturation unknown) in 10% EtOH + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear EtOH stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 5: ≥ 2.5 mg/mL (7.74 mM) (saturation unknown) in 10% EtOH + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear EtOH stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 6: ≥ 2.5 mg/mL (7.74 mM) (saturation unknown) in 10% EtOH + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear EtOH stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 7: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (7.74 mM) Solubility in Formulation 8: 2.5 mg/mL (7.74 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0947 mL | 15.4736 mL | 30.9473 mL | |
| 5 mM | 0.6189 mL | 3.0947 mL | 6.1895 mL | |
| 10 mM | 0.3095 mL | 1.5474 mL | 3.0947 mL |