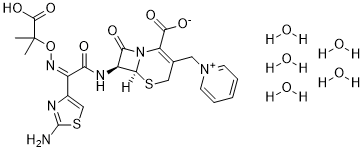
Ceftazidime hydrate (Fortaz; Fortum; GR20263; LY-139381; GR-20263), the hydrated form of Ceftazidime, is a 3rd generation, broad-spectrum β-lactam antibiotic/cephalosporin antibiotic useful for the treatment of a number of bacterial infections.
Physicochemical Properties
| Exact Mass | 546.099 |
| Elemental Analysis | C, 41.51; H, 5.07; N, 13.20; O, 30.16; S, 10.07 |
| CAS # | 78439-06-2 |
| Related CAS # | Ceftazidime;72558-82-8 |
| PubChem CID | 6536864 |
| Appearance | White to off-white solid powder |
| Melting Point | >150ºC(dec.) |
| LogP | -2.84 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 17 |
| Rotatable Bond Count | 8 |
| Heavy Atom Count | 42 |
| Complexity | 1020 |
| Defined Atom Stereocenter Count | 2 |
| SMILES | CC(C)(C(=O)O)O/N=C(/C1=CSC(=N1)N)\C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)C[N+]4=CC=CC=C4)C(=O)[O-] |
| InChi Key | NMVPEQXCMGEDNH-CYWOSJMDSA-N |
| InChi Code | InChI=1S/C22H22N6O7S2.5H2O/c1-22(2,20(33)34)35-26-13(12-10-37-21(23)24-12)16(29)25-14-17(30)28-15(19(31)32)11(9-36-18(14)28)8-27-6-4-3-5-7-27/h3-7,10,14,18H,8-9H2,1-2H3,(H4-,23,24,25,29,31,32,33,34)5*1H2/b26-13-/t14-,18-/m0...../s1 |
| Chemical Name | (6S,7S)-7-((Z)-2-(2-aminothiazol-4-yl)-2-(((2-carboxypropan-2-yl)oxy)imino)acetamido)-8-oxo-3-(pyridin-1-ium-1-ylmethyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate pentahydrate |
| Synonyms | Ceftazidime; Anhydrous, Ceftazidime;Ceftazidime; Ceftazidime Anhydrous; Ceftazidime Pentahydrate; Fortaz; Fortum; GR 20263; GR-20263; GR20263; LY 139381; LY-139381; LY139381; Pentahydrate, Ceftazidime; Tazidime; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam |
| ln Vitro |
Antibacterial and anti-biofilm properties against P. aeruginosa strains are demonstrated by ceftazidime (0-8 µg/mL, approx., 24 h) pentahydrate[2]. Ceftazidime pentahydrate (0–40 µg/mL, approximately; 18–20 h) exhibits inhibitory effects on isolates of S. maltophilia[3]. |
| ln Vivo | In a murine thigh infection model, ceftazidime (2 h infusion of injection, 2 000 mg, every 8 h for 24 h) pentahydrate moderately reduces bacterial density[4]. |
| Cell Assay |
Cell Line: P. aeruginosa strains (PAO1, PA1, PA2) Concentration: 0-8 µg/mL approximately Incubation Time: 24 h Result: demonstrated MIC values of 2-4 µg/mL for antibacterial and anti-biofilm activities. |
| Animal Protocol |
Animal Model: Murine thigh infection model[4] Dosage: 2000 mg Administration: 2 h infusion of injection, every 8 h for 24 h. Result: decreased bacterial density when compared to the isogenic strain of NDM (New Delhi metallo-β-lactamase). |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that ceftazidime produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Avibactam has not been studied in nursing mothers. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Ceftazidime-avibactam is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. ◉ Summary of Use during Lactation Limited information indicates that ceftazidime produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Ceftazidime and is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| References |
[1]. Ceftazidime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1985 Feb;29(2):105-61. [2]. In vitro activities of cellulase and ceftazidime, alone and in combination against Pseudomonas aeruginosa biofilms. BMC Microbiol. 2021 Dec 16;21(1):347. [3]. Avibactam potentiated the activity of both ceftazidime and aztreonam against S. maltophilia clinical isolates in vitro. BMC Microbiol. 2021 Feb 22;21(1):60. [4]. Unexpected in vivo activity of ceftazidime alone and in combination with avibactam against New Delhi metallo-β-lactamase-producing Enterobacteriaceae in a murine thigh infection model. Antimicrob Agents Chemother. 2014 Nov;58(11):7007-9. |
| Additional Infomation |
Ceftazidime pentahydrate is a hydrate that is the pentahydrate of ceftazidime, a cephalosporin having 7beta-[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-{[(2-carboxypropan-2-yl)oxy]imino}acetyl]amino and 3-pyridinium-1-ylmethyl side-groups. It contains a ceftazidime. Semisynthetic, broad-spectrum antibacterial derived from CEPHALORIDINE and used especially for Pseudomonas and other gram-negative infections in debilitated patients. |
Solubility Data
| Solubility (In Vitro) |
DMSO : 2~100 mg/mL (3.65~157.07 mM) Water : ~25 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (3.93 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (3.93 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |